Using the result obtained in the pages 4 and 5, answer the part 1 and part 2 questions in detail, along with the lab report ( including purpose, expectations, observations/results, sources of error - not error made by people performing the lab - and a discusion ) ASAP PLEASE

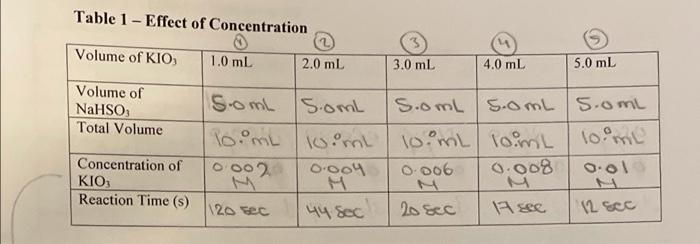
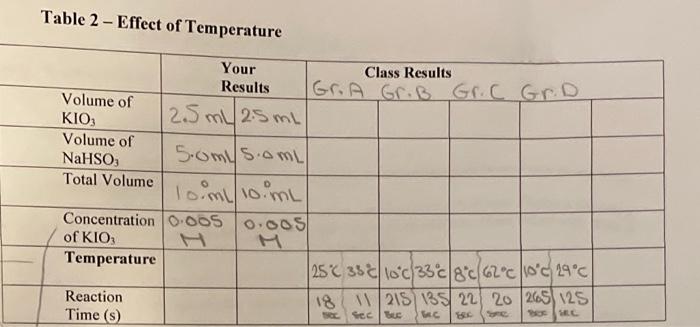
PROCEDURE Partl Effect of Concentration 5.0 1. Put on your lab apron and safety goggles. 30 45 2. Obtain approximately 60 mL of Solution A 10.02M KIO,) and mlof Solution 300.002M NaHSO, containing H,SO, and starch) In 100 ml beakers S. 3. In a 10 ml graduated cylinder place 100 ml of Solution A using * medicine dropper to obtain the volume as accurately as possible. Transfer the solution to an 18 mm 150 mm test tube in a rack 4. In the same manner measure out 10 mL of Solution Band transfer it to another test tube. Use a different graduated cylinder and medicine dropper than for Solution A and keep the same ones for each solution for subsequent parts of the procedure. 5. In order to measure the time needed for the reaction to occur, you will need a watch or clock with a sweep second hand, or preferably digital watch with a stopwatch function. One partner must record the time while the other partner mixes the solutions. Mix the solutions in one of the two test tubes, and record the time from the instant they first mix 8. Very quickly pour the solution back and forth between the two test tubes three times to make sure they are thoroughly mixed, then wall for the completion of the reaction. 7. Record the time at the Instant the deep blue-black color first appears 8. In order to study the effect of changing the concentration of Soleil tion he class will now be assigned the yalues Whem. and a mi. In each Instance measure out the volume in thegraduated cylinder, and add enough water to make it up to the mi mark Den transfer each dilution of Solution Atoa test tube and mix it with mL of solution Bas before. For each, record the time taken for the color to appear in Table 1 in your notebook 9. Record your results on the board in order to get class averages Bxperiment 180 ml. 198 Partil Effect of Temperature 1. In this part of the procedure you will keep the concentration constant, and vary the temperature, both above and below room temperature. In order that the observed reaction times lie in a suitable range, Solution will be at only half the concentration of the original solution. RT 2. Make ypsets of each of solutops A and 8, with mL of Solution A and mL of yeater in one set oftest tubes, and mL of Solution B in the other test tubes. soo 3. Make 4 water baths (in 20 ml boakers) at teroportures ofobie, any two temperatures under 5c. 296.GetG 200C, E ander depending on which seto temperature your underassignyouse ice to obtain the tempera- tures below room temperature, and hot water from a tap or kettle to obtain the temperatures above room temperature. The beakers should be about two thirds full, so that the solutions in the test tubes are well beneath the water in the baths. 4. Place one test tube containing diluted Solution A and another con talning Solution B in each water bath, and leave them for 10 min to allow them to adjust to the temperature required. 5. Try to maintain the temperatures in the water baths within 0.s Cofthe temperatures assigned to make comparisons with other groups in the class more meaningful 6. When the temperatures are at the correct value, and the tubes have been in for enough time, mix each pair of solutions in one test tube by pouring them back and forth three times. Then place that test tube back in the water bath. In Table 2, record the time from the instant the solutions are mixed to the first appearance of the blue-black color. Do this for each pair of solutions at each temperature. 7. Hered your results on the bound in order to get class averages Record your results on the board & record all class results. + POST LAB DISCUSSION The blue black color observed in Parts I and II oscura na rafult of two separate reactions. Initially, the lodale ion, 105, reactswithabletelon HSOs, giving lodido lon, 1, and sulfate lon, Sot, as follows: 10slaq) + 3H805laq) rlaq) + 380 laq) + 3H00 The blaukite ions are present in lower concentration and are, therefore, used up first. When this happens, the log fons than react with lions in the presence of Hions to give molecular lodine, L: 1Ojlaq) + 5rlaql + 6H'laq - 3Llaq) + 3H2011) In the presence of starch, lodine forms the intense blue-black color as a result of the lodlne molecules being trapped in the long starch molecules. The appearance of this color indicates that the first reaction is complete and the second one has begun to take place. The uncertainty involved in measuring the time taken for the reaction could easily be t2 sunless you are very careful. Thus the interpretation of results will be more meaningful if the class averages at a particular concen reaction to be completed is not the same as measuring its rate, but there is an Inverse relation between them: rate is proportional to the reciprocal of time. Consequently, in interpreting the results you will calculate the rate in terms of reciprocal seconds (8-1) and plot graphs of the rate against con- centration or temperature. Table 1 - Effect of Concentration - Volume of KIO 1.0 mL 2.0 mL 3.0 mL 4.0 mL 5.0 mL Volume of NaHSO Total Volume Concentration of KIO Reaction Time (s) S.om somL S.omL S.OmL 5.0L Toimit loimL 10 mL loimL 10 ml 0002 0.004 0.006 0.008 | 0.01 44 sec 20 sec 19 sec. 12 sec 1120 sec Table 2 - Effect of Temperature Your Class Results Results Gr. A Gr.B Gr.C Gr.D. Volume of KIO 25 ml 2.5ml Volume of NaHSO, 5.omis.oml Total Volume lo.ml/10 mL Concentration 0.005 0.00 of KIO Temperature | 258 35c10c/33c/8 c/620/1000/29c) Reaction 18 11/215) 135/22) 20/265/125 Time () Seco BE SEC Follow-up to the lodine Clock Experiment Note: Omit Questions that require the class averages. QUESTIONS AND CALCULATIONS Partl Effect of Concentration 1. Calculate the concentration of KIO, in the final mixture for each of your dilutions. Remember Solution original [K10.) was 0.02m, and that the original dilution, when mixed with 10 mil.com Solution B. gave a total volume of 20 ml in each case. Calculate the rate of the reaction in st) for each case. 9. Plot a graph of your results, with rate plotted against (KIO.). Repeat these calculations with the class averages, and plot the results on the same sheet of graph paper as for question 3. Remember to label your results and the class averages. 5. Considering any uncertainties in time for the reaction, what type of graph results? 6. Refeming to the collision theory of reaction rates, state why you would expect the change of rate with concentration that you observed. Part II Effect of Temperature Calculate the rate of the reaction at each temperature lins") by taking the reciprocal of the time elapsed 2. Calculate the factor by which the rate changed for each 10'C interval in temperature. 3. Plot a graph of the results, with rate plotted against temperature. Repeat these calculations using the class averages, and plot the results on the same graph paper. 5. Referring to the collision theory of reaction rates, state why you would expect the change of rate with temperature that you observed. Purpose (1): The purpose of this lab is to observe te effect of temprature and concentration on the rate of a chemical reaction. Expectation(s) (2): Materials (2): Reagents) 1x Batey goggles per person 2 bankas loomL) solution A = 0.02M klog 2 graduted cylinders (loml) solution B +0.002 M Natsay Apparatus 1x thermometer per group containing 4g of starch 2 medicine droppers and 12mL of M Hisoy/h) Ice Camount depindo) 6 Toot tubes (igmmx isomm) (1x clock/watch Tap on hot water camount deponds) Observations/Results (4): Sources of Error (2): Discussion: (3)