Using the tables below from Perry's chemical engineering handbook determine the following:
- The viscosity of diethyl ether (in Pascal-second) at 77 degrees Celsius
- Viscosity (Pascal-second) of cyclohexene at 50 degrees Celsius.
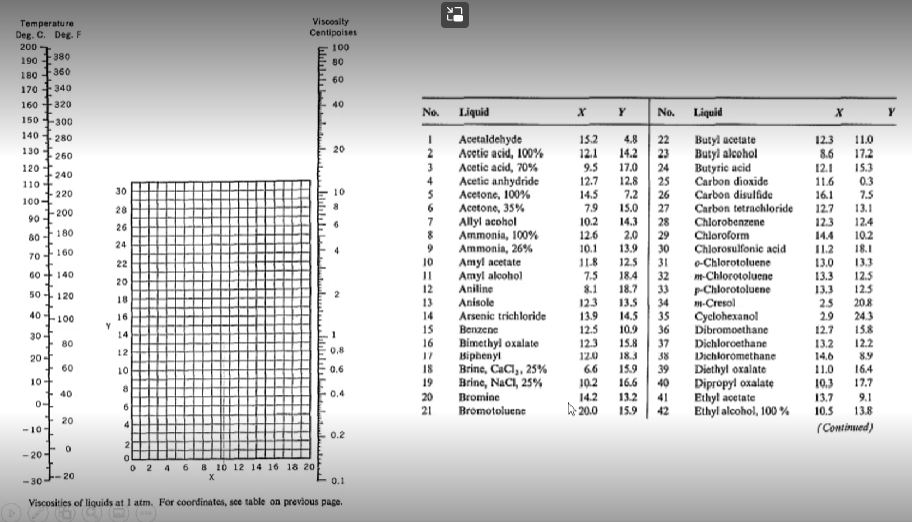
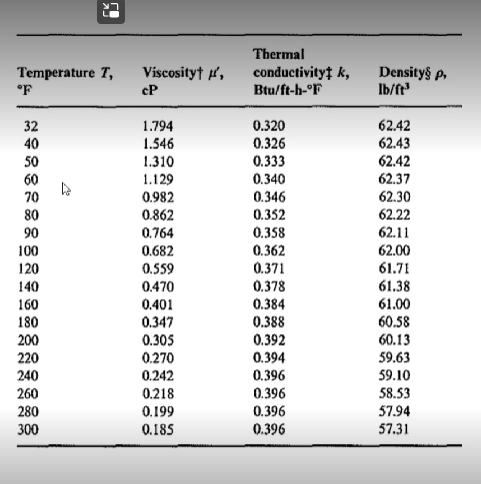
22 Temperature Deg. C. Deg. F 200 190 180 -360 170 - 340 160 +320 Viscosity Centipoises 100 80 60 -380 40 X Y Y 150-300 280 140 130 20 260 120 110 152 12.1 9.5 12.7 14.5 240 220 30 100- 90 200 79 28 26 80 180 24 4.8 14.2 17.0 12.8 7.2 15.0 14.3 2.0 13.9 12.5 18.4 18.7 13.5 14.5 10.9 70 160 No. Liquid Acetaldehyde Acetic acid, 100% Acetic acid, 70% 4 Acetic anhydride 5 Acetone, 100% Acetone, 35% 7 Allyl acohol 8 Ammonia, 100% 9 Ammonia, 26% Amyl acetate 11 Amyl alcohol 12 Aniline 13 Anisole 14 Arsenic trichloride 15 | Benzema 16 Bimethyl oxalate 17 Biphenyi 18 Brinc, CaCl,. 25% 19 Brinc, NaCl, 25% 20 Bromine 21 Bromotoluenc 22 No. Liquid 22 Butyl acetate 23 Butyl alcohol 24 Butyric acid 25 Carbon dioxide 26 Carbon disulfide 27 Carbon tetrachloride 28 Chlorobenzene 29 Chloroform 30 Chlorosulfonic acid 31 o-Chlorotoluene 32 m-Chlorotoluene 33 p-Chlorotoluene 34 1H-Cresol 35 Cyclohexanol 36 Dibromoethane 37 Dichloroethane 38 Dichloromethane 39 Diethyl oxalate 40 Dipropyl oxalate 41 Ethyl acetate 42 Ethyl alcohol, 100% 60+ 140 20 12.3 11.0 8.6 17.2 12.1 15.3 11.6 03 16.1 7.5 12.7 13.1 12.3 12.4 14.4 102 IL2 18.1 13.0 13.3 13.3 12.5 13.3 125 2.5 20.8 29 243 12.7 15.8 13.2 122 14,6 8.9 11.0 16.4 10.3 17.7 13.7 9.1 13.8 (Continued) 50 120 10.2 12.6 10.1 11.8 7.5 8.1 123 13.9 12.5 123 120 6.6 10.2 14.2 h 20.0 18 40-100 16 Y 14 15.8 12 0,8 30-1 80 20-1 60 10+ 40 0+ 10 0.6 183 15.9 16.6 132 15.9 8 0.4 & 6 10.5 20 4 -101 0.2 0 -20-1 2 4 6 8 10 12 14 16 18 20 -30 20 0.1 Viscosities of liquids at 1 atm. For coordinates, see table on previous page. Temperature T, OF Viscosityti, cP Thermal conductivity k, Btu/ft-b-or Densitys Ib/ft? 32 40 50 60 70 80 90 100 120 140 160 180 200 220 240 260 280 300 1.794 1.546 1.310 1.129 0.982 0.862 0.764 0.682 0.559 0.470 0.401 0.347 0.305 0.270 0.242 0.218 0.199 0.185 0.320 0.326 0.333 0.340 0.346 0.352 0.358 0.362 0.371 0.378 0.384 0.388 0.392 0.394 0.396 0.396 0.396 0.396 62.42 62.43 62.42 62.37 62.30 62.22 62.11 62.00 61.71 61.38 61.00 60.58 60.13 59.63 59.10 58.53 57.94 57.31 22 Temperature Deg. C. Deg. F 200 190 180 -360 170 - 340 160 +320 Viscosity Centipoises 100 80 60 -380 40 X Y Y 150-300 280 140 130 20 260 120 110 152 12.1 9.5 12.7 14.5 240 220 30 100- 90 200 79 28 26 80 180 24 4.8 14.2 17.0 12.8 7.2 15.0 14.3 2.0 13.9 12.5 18.4 18.7 13.5 14.5 10.9 70 160 No. Liquid Acetaldehyde Acetic acid, 100% Acetic acid, 70% 4 Acetic anhydride 5 Acetone, 100% Acetone, 35% 7 Allyl acohol 8 Ammonia, 100% 9 Ammonia, 26% Amyl acetate 11 Amyl alcohol 12 Aniline 13 Anisole 14 Arsenic trichloride 15 | Benzema 16 Bimethyl oxalate 17 Biphenyi 18 Brinc, CaCl,. 25% 19 Brinc, NaCl, 25% 20 Bromine 21 Bromotoluenc 22 No. Liquid 22 Butyl acetate 23 Butyl alcohol 24 Butyric acid 25 Carbon dioxide 26 Carbon disulfide 27 Carbon tetrachloride 28 Chlorobenzene 29 Chloroform 30 Chlorosulfonic acid 31 o-Chlorotoluene 32 m-Chlorotoluene 33 p-Chlorotoluene 34 1H-Cresol 35 Cyclohexanol 36 Dibromoethane 37 Dichloroethane 38 Dichloromethane 39 Diethyl oxalate 40 Dipropyl oxalate 41 Ethyl acetate 42 Ethyl alcohol, 100% 60+ 140 20 12.3 11.0 8.6 17.2 12.1 15.3 11.6 03 16.1 7.5 12.7 13.1 12.3 12.4 14.4 102 IL2 18.1 13.0 13.3 13.3 12.5 13.3 125 2.5 20.8 29 243 12.7 15.8 13.2 122 14,6 8.9 11.0 16.4 10.3 17.7 13.7 9.1 13.8 (Continued) 50 120 10.2 12.6 10.1 11.8 7.5 8.1 123 13.9 12.5 123 120 6.6 10.2 14.2 h 20.0 18 40-100 16 Y 14 15.8 12 0,8 30-1 80 20-1 60 10+ 40 0+ 10 0.6 183 15.9 16.6 132 15.9 8 0.4 & 6 10.5 20 4 -101 0.2 0 -20-1 2 4 6 8 10 12 14 16 18 20 -30 20 0.1 Viscosities of liquids at 1 atm. For coordinates, see table on previous page. Temperature T, OF Viscosityti, cP Thermal conductivity k, Btu/ft-b-or Densitys Ib/ft? 32 40 50 60 70 80 90 100 120 140 160 180 200 220 240 260 280 300 1.794 1.546 1.310 1.129 0.982 0.862 0.764 0.682 0.559 0.470 0.401 0.347 0.305 0.270 0.242 0.218 0.199 0.185 0.320 0.326 0.333 0.340 0.346 0.352 0.358 0.362 0.371 0.378 0.384 0.388 0.392 0.394 0.396 0.396 0.396 0.396 62.42 62.43 62.42 62.37 62.30 62.22 62.11 62.00 61.71 61.38 61.00 60.58 60.13 59.63 59.10 58.53 57.94 57.31








