Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Vinegar analysis lab help fill the chart in!! Molarity of NaOH is 0.4937 M begin{tabular}{|l|l|l|l|} hline & Trial 1 & Trial 2 & Trial 3
Vinegar analysis lab help fill the chart in!! 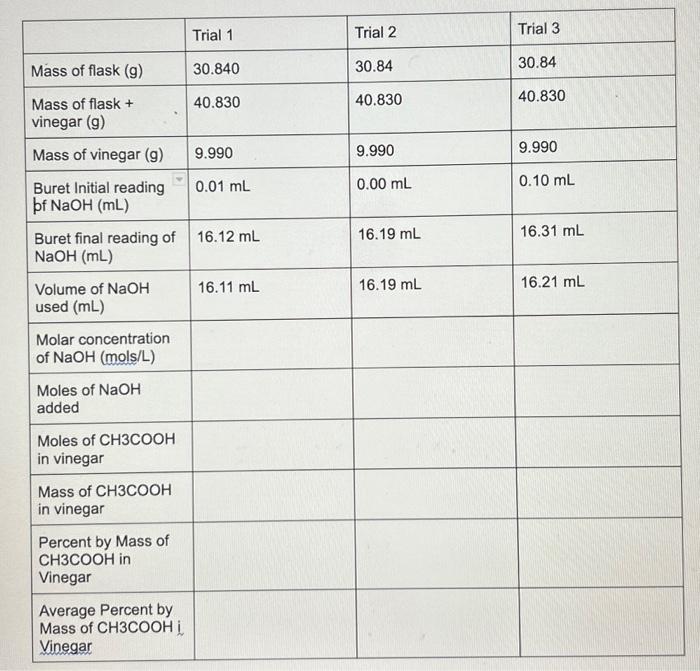

Molarity of NaOH is 0.4937 M
\begin{tabular}{|l|l|l|l|} \hline & Trial 1 & Trial 2 & Trial 3 \\ \hline Mass of flask (g) & 30.840 & 30.84 & 30.84 \\ \hline Massofflask+vinegar(g) & 40.830 & 40.830 & 40.830 \\ \hline Mass of vinegar (g) & 9.990 & 9.990 & 9.990 \\ \hline BuretInitialreadingpfNaOH(mL) & 0.01mL & 0.00mL & 0.10mL \\ \hline BuretfinalreadingofNaOH(mL) & 16.12mL & 16.19mL & 16.31mL \\ \hline VolumeofNaOHused(mL) & 16.11mL & 16.19mL & \\ \hline MolarconcentrationofNaOH(mols/L) & & & \\ \hline MolesofNaOHadded & & & \\ \hline MolesofCH3COOHinvinegar & & & \\ \hline MassofCH3COOHinvinegar & & & \\ \hline PercentbyMassofCH3COOHinVinegar & & & \\ \hline AveragePercentbyMassofCH3COOHVinegar & & & \\ \hline \end{tabular} Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


