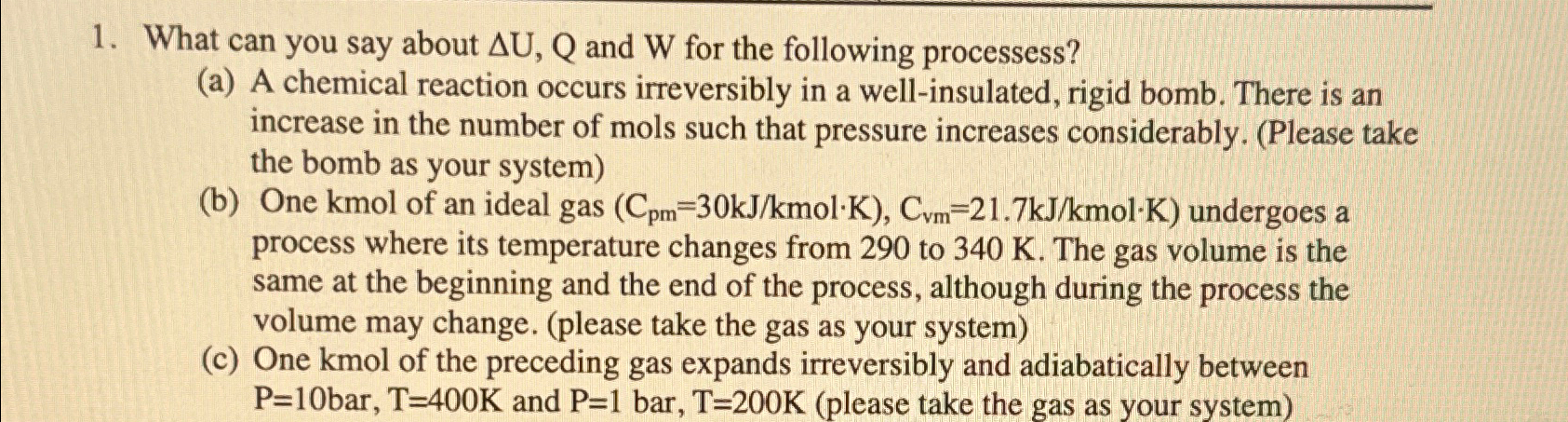
Question
What can you say about Delta U,Q and W for the following processess? (a) A chemical reaction occurs irreversibly in a well-insulated, rigid bomb. There
What can you say about
\\\\Delta U,Qand
Wfor the following processess?\ (a) A chemical reaction occurs irreversibly in a well-insulated, rigid bomb. There is an increase in the number of mols such that pressure increases considerably. (Please take the bomb as your system)\ (b) One
kmolof an ideal gas (
{(
:C_(pm)=30(kJ)/(k)mol*K),C_(vm)=21.7(kJ)/(k)mol*K} undergoes a process where its temperature changes from 290 to
340K. The gas volume is the same at the beginning and the end of the process, although during the process the volume may change. (please take the gas as your system)\ (c) One kmol of the preceding gas expands irreversibly and adiabatically between
P=(10)/(b)ar ,T=400Kand
P=(1)/(b)ar ,T=200K(please take the gas as your system)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


