Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) A thermal plant in Australia utilizes locally sourced coal for power generation. This particular coal is found to contain ash 9.4%, hydrogen 6%,
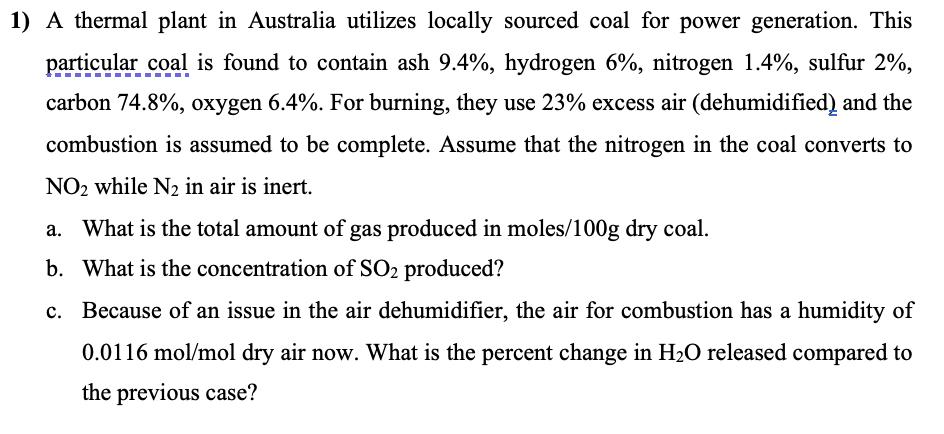
1) A thermal plant in Australia utilizes locally sourced coal for power generation. This particular coal is found to contain ash 9.4%, hydrogen 6%, nitrogen 1.4%, sulfur 2%, carbon 74.8%, oxygen 6.4%. For burning, they use 23% excess air (dehumidified) and the combustion is assumed to be complete. Assume that the nitrogen in the coal converts to NO2 while N2 in air is inert. What is the total amount of gas produced in moles/100g dry coal. b. What is the concentration of SO2 produced? c. Because of an issue in the air dehumidifier, the air for combustion has a humidity of 0.0116 mol/mol dry air now. What is the percent change in HO released compared to the previous case?
Step by Step Solution
★★★★★
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
To determine the most stable carbocation we need to consider the stability of the carbocation i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


