Question
Which of the following represents the most stable conformation for propane? Which of the following compounds have N atom with delocalized electrons? In its launching
Which of the following represents the most stable conformation for propane?
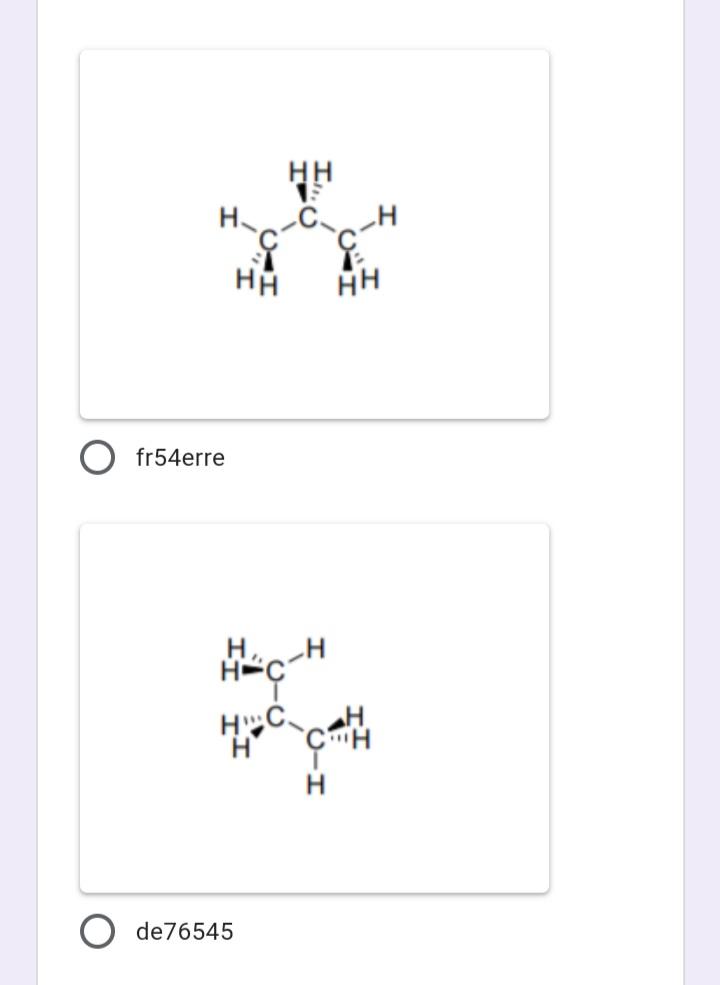
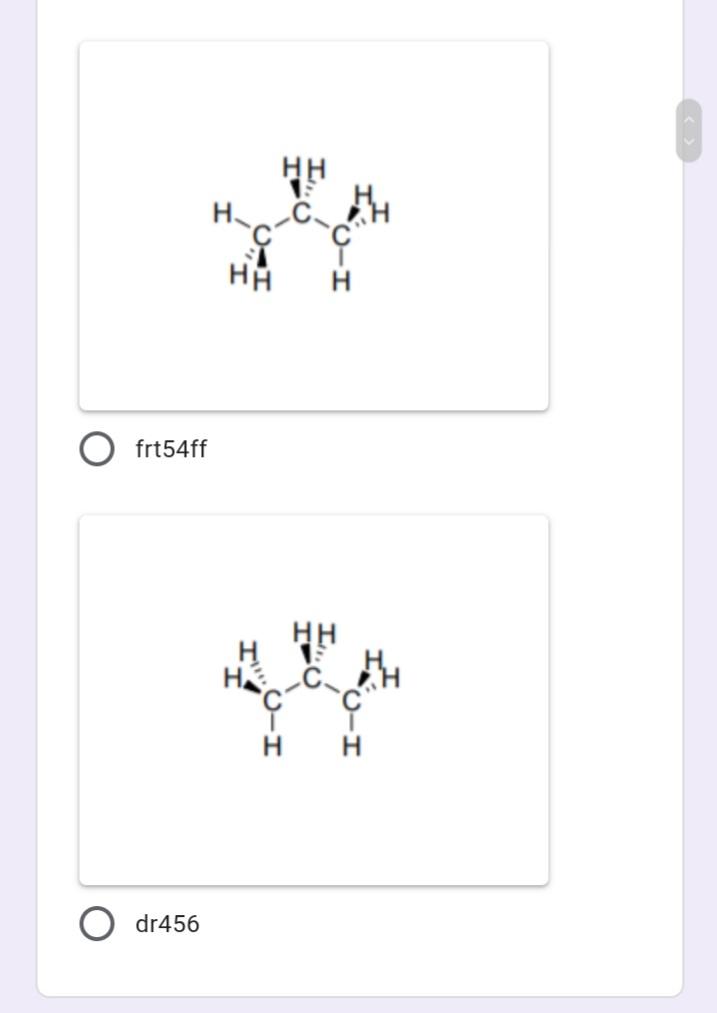
Which of the following compounds have N atom with delocalized electrons?

In its launching year, the drug (structure below) was used by over three million satisfied customers. Determine the number of chiral carbons in this drug.
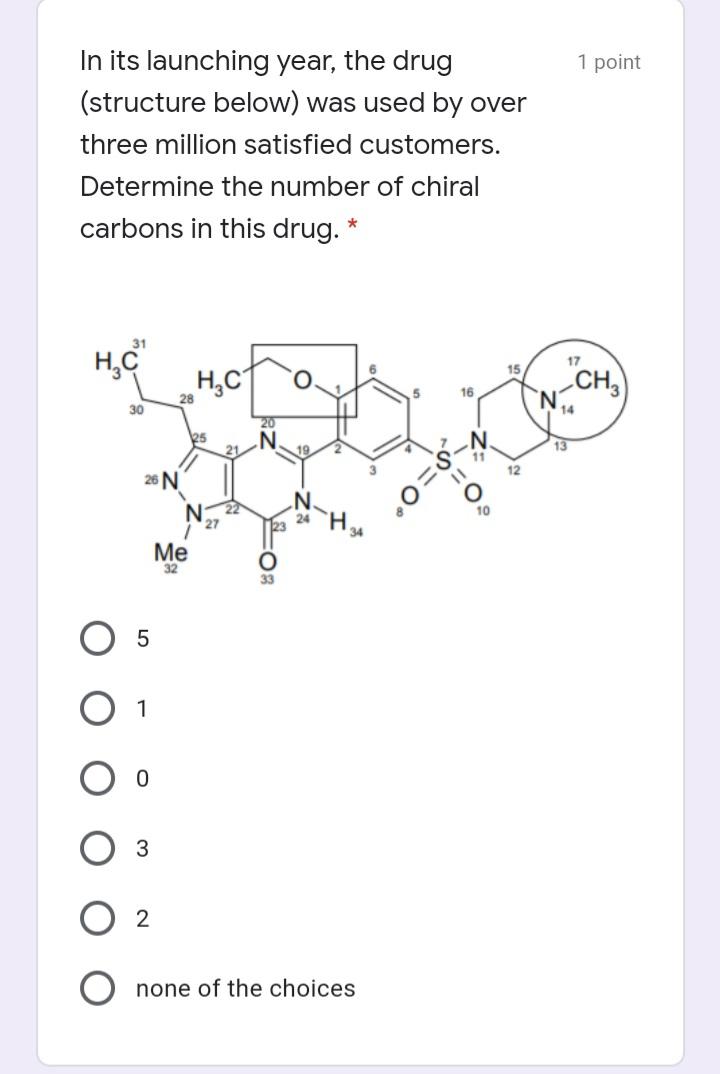

HH fr54erre H.H HCH H de76545
Step by Step Solution
3.57 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
1 The most stable conformer of propane is that in which all adjoining carbon atom are ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Marketing
Authors: Dhruv Grewal, Michael Levy
6th edition
1259709078, 9781259924033, 978-1259709074
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



