Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Why is it your experts are saying H2 + 2S = 2H.S is the step 3 in the mechanism below. Please work out the question
Why is it your experts are saying H2 + 2S = 2H.S is the step 3 in the mechanism below. Please work out the question nicely, l am not satisfied with the answer given if you say step 3 is H2 + 2S = 2H.S. Please work out upto the final answer. explain each and every step
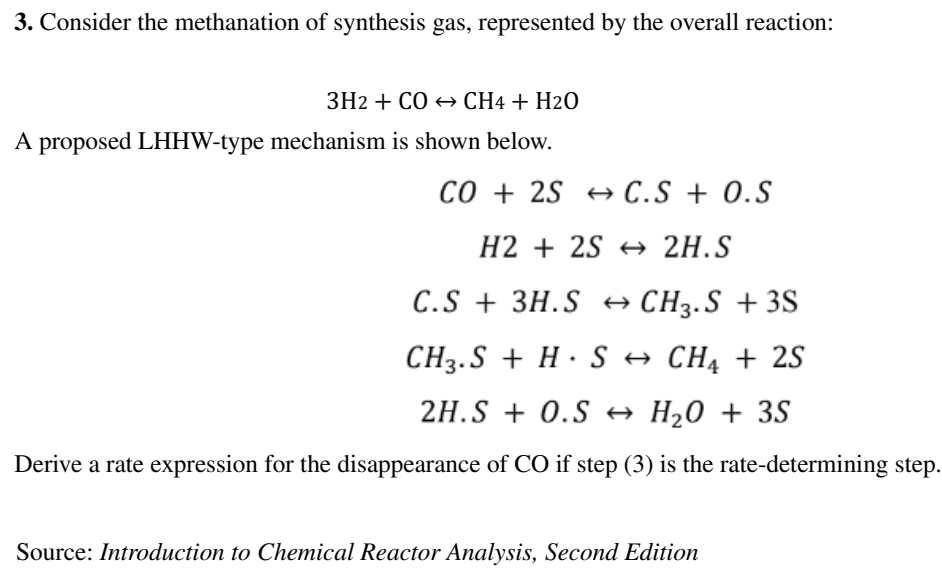 3. Consider the methanation of synthesis gas, represented by the overall reaction: 3H2+COCH4+H2O A proposed LHHW-type mechanism is shown below. CO+2SC.S+O.SH2+2S2HSC.S+3H.SCH3.S+3SCH3.S+HSCH4+2S2H.S+O.SH2O+3S Derive a rate expression for the disappearance of CO if step (3) is the rate-determining step. Source: Introduction to Chemical Reactor Analysis, Second Edition 3. Consider the methanation of synthesis gas, represented by the overall reaction: 3H2+COCH4+H2O A proposed LHHW-type mechanism is shown below. CO+2SC.S+O.SH2+2S2HSC.S+3H.SCH3.S+3SCH3.S+HSCH4+2S2H.S+O.SH2O+3S Derive a rate expression for the disappearance of CO if step (3) is the rate-determining step. Source: Introduction to Chemical Reactor Analysis, Second Edition
3. Consider the methanation of synthesis gas, represented by the overall reaction: 3H2+COCH4+H2O A proposed LHHW-type mechanism is shown below. CO+2SC.S+O.SH2+2S2HSC.S+3H.SCH3.S+3SCH3.S+HSCH4+2S2H.S+O.SH2O+3S Derive a rate expression for the disappearance of CO if step (3) is the rate-determining step. Source: Introduction to Chemical Reactor Analysis, Second Edition 3. Consider the methanation of synthesis gas, represented by the overall reaction: 3H2+COCH4+H2O A proposed LHHW-type mechanism is shown below. CO+2SC.S+O.SH2+2S2HSC.S+3H.SCH3.S+3SCH3.S+HSCH4+2S2H.S+O.SH2O+3S Derive a rate expression for the disappearance of CO if step (3) is the rate-determining step. Source: Introduction to Chemical Reactor Analysis, Second Edition Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


