Answered step by step
Verified Expert Solution
Question
1 Approved Answer
write the balanced chemical equation and fill in the blanks Trial 2 Trial 3 25 500 25 25 0.02002 Table 3. Determination of H202 in
write the balanced chemical equation and fill in the blanks 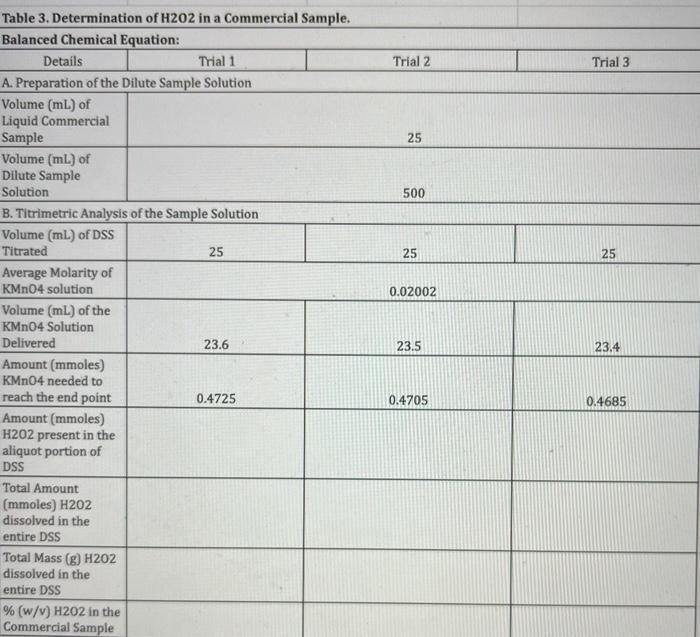
Trial 2 Trial 3 25 500 25 25 0.02002 Table 3. Determination of H202 in a Commercial Sample. Balanced Chemical Equation: Details Trial 1 A. Preparation of the Dilute Sample Solution Volume (ml.) of Liquid Commercial Sample Volume (ml) of Dilute Sample Solution B. Titrimetric Analysis of the Sample Solution Volume (ml.) of DSS Titrated 25 Average Molarity of KMnO4 solution Volume (mL) of the KMnO4 Solution Delivered 23.6 Amount (mmoles) KMnO4 needed to reach the end point 0.4725 Amount (mmoles) H202 present in the aliquot portion of DSS Total Amount (mmoles) H202 dissolved in the entire DSS Total Mass (g) H202 dissolved in the entire DSS % (w/v) H202 in the Commercial Sample 23.5 23.4 0.4705 0.4685 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


