Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Write the material balance equation for part a and part b as the guideline in the picture above CO+H2OCO2+H2 In the reactor system shown in
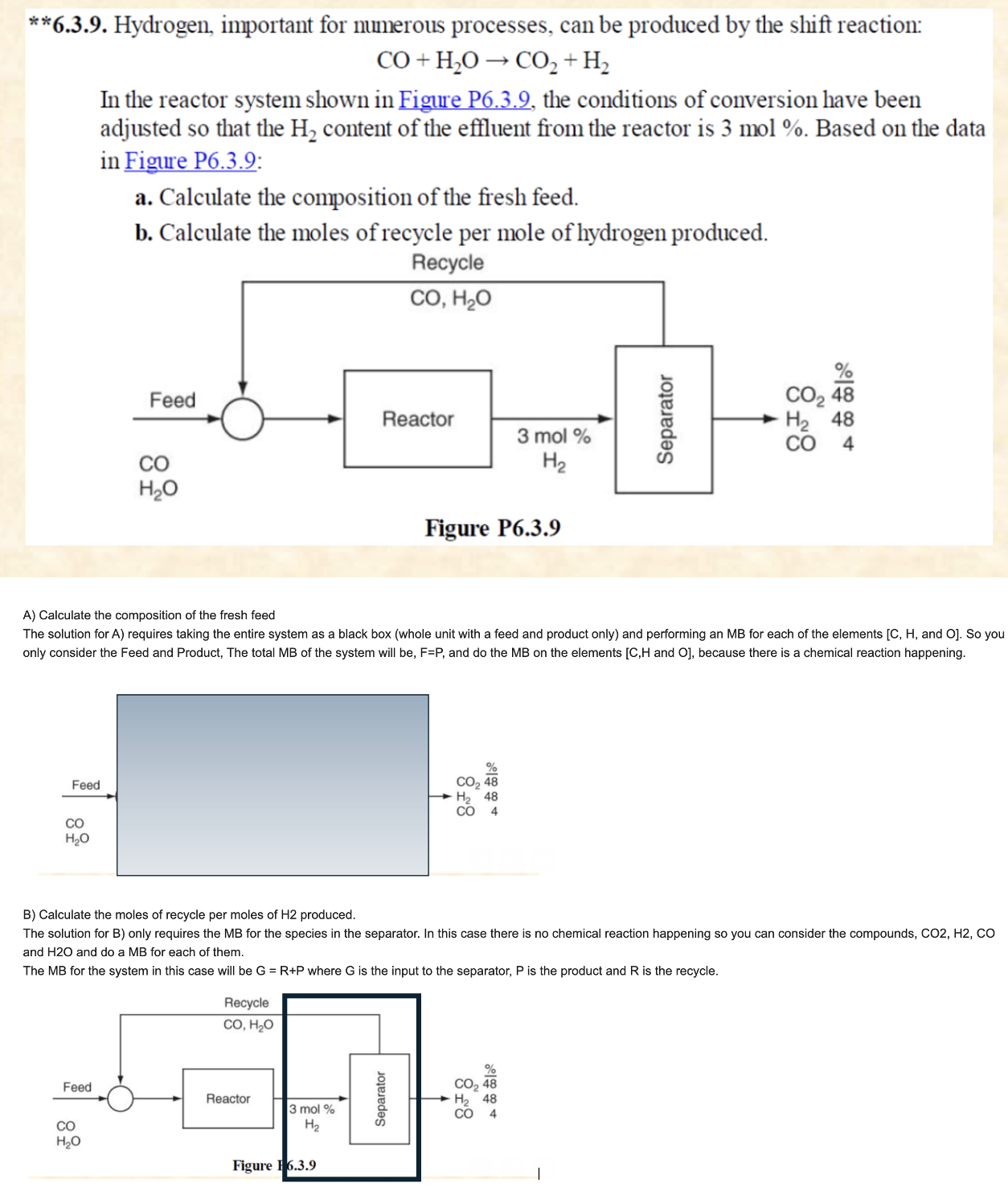
Write the material balance equation for part a and part b as the guideline in the picture above
CO+H2OCO2+H2 In the reactor system shown in Figure P6.3.9, the conditions of conversion have been adjusted so that the H2 content of the effluent from the reactor is 3mol%. Based on the data in Figure P6.3.9: a. Calculate the composition of the fresh feed. b. Calculate the moles of recycle per mole of hydrogen produced. A) Calculate the composition of the fresh feed The solution for A ) requires taking the entire system as a black box (whole unit with a feed and product only) and performing an MB for each of the elements [C, H, and O ]. So you only consider the Feed and Product, The total MB of the system will be, F=P, and do the MB on the elements [ C,H and O], because there is a chemical reaction happening. B) Calculate the moles of recycle per moles of H2 produced. The solution for B ) only requires the MB for the species in the separator. In this case there is no chemical reaction happening so you can consider the compounds, CO2,H2,CO and H2O and do a MB for each of them. The MB for the system in this case will be G=R+P where G is the input to the separator, P is the product and R is the recycleStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


