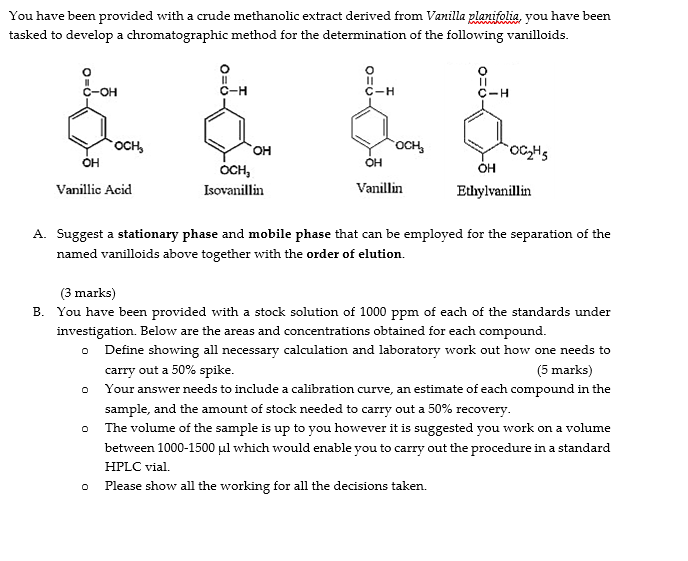
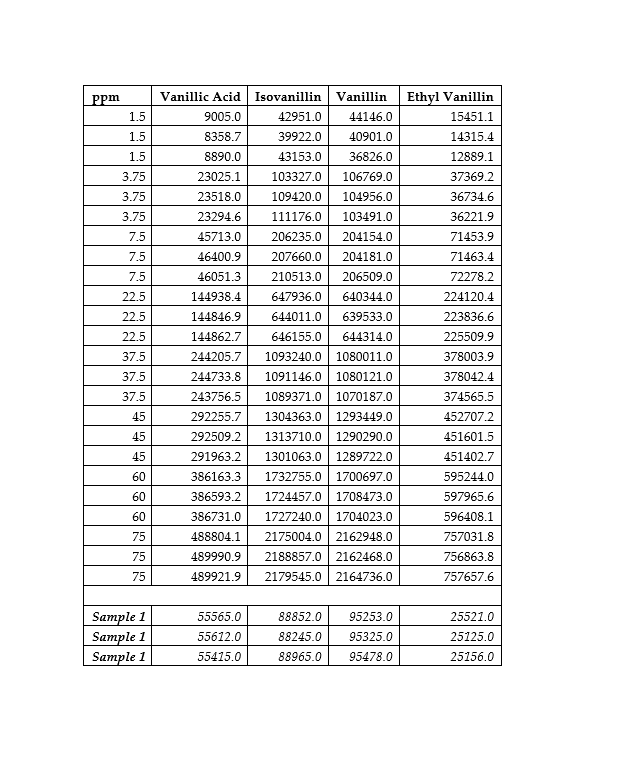
You have been provided with a crude methanolic extract derived from Vanilla planifolia, you have been tasked to develop a chromatographic method for the determination of the following vanilloids. 11 C-H 0 11 C-H C-OH C-H OCH OCH, OH OH OCH, Isovanillin -OGhs OH Vanillic Acid Vanillin Ethylvanillin A. Suggest a stationary phase and mobile phase that can be employed for the separation of the named vanilloids above together with the order of elution. (3 marks) B. You have been provided with a stock solution of 1000 ppm of each of the standards under investigation. Below are the areas and concentrations obtained for each compound. Define showing all necessary calculation and laboratory work out how one needs to carry out a 50% spike. (5 marks) Your answer needs to include a calibration curve, an estimate of each compound in the sample, and the amount of stock needed to carry out a 50% recovery. The volume of the sample is up to you however it is suggested you work on a volume between 1000-1500 ul which would enable you to carry out the procedure in a standard HPLC vial. Please show all the working for all the decisions taken. 0 0 ppm 1.5 1.5 1.5 3.75 3.75 Vanillic Acid 9005.0 8358.7 8890.0 23025.1 23518.0 23294.6 45713.0 46400.9 3.75 7.5 Ethyl Vanillin 15451.1 14315.4 12889.1 37369.2 36734.6 36221.9 71453.9 71463.4 72278.2 224120.4 223836.6 225509.9 378003.9 378042.4 7.5 7.5 46051.3 144938.4 22.5 22.5 144846.9 22.5 37.5 Isovanillin Vanillin 42951.0 44146.0 39922.0 40901.0 43153.0 36826.0 103327.0 106769.0 109420.0 104956.0 111176.0 103491.0 206235.0 204154.0 207660.0 204181.0 210513.0 206509.0 647936.0 640344.0 644011.0 639533.0 646155.0 644314.0 1093240.0 1080011.0 1091146.0 1080121.0 1089371.0 1070187.0 1304363.0 1293449.0 1313710.0 1290290.0 1301063.0 1289722.0 1732755.0 1700697.0 1724457.0 1708473.0 1727240.0 1704023.0 2175004.0 2162948.0 2188857.0 2162468.0 2179545.0 2164736.0 144862.7 244205.7 37.5 244733.8 243756.5 374565.5 37.5 45 292255.7 45 45 60 60 292509.2 291963.2 386163.3 386593.2 386731.0 488804.1 489990.9 489921.9 452707.2 451601.5 451402.7 595244.0 597965.6 596408.1 757031.8 756863.8 757657.6 60 75 75 75 Sample 1 Sample 1 Sample 1 55565.0 55612.0 55415.0 88852.0 88245.0 88965.0 95253.0 95325.0 95478.0 25521.0 25125.0 25156.0








