Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You need to specify which unit you are considering when solving to calculate the composition of CO2 and H2O in the feed. Additionally you need
You need to specify which unit you are considering when solving to calculate the composition of CO2 and H2O in the feed. Additionally you need to specify which unit you are considering and list the Mass balance equations for that unit when solving for b. In any case you have the right solution,
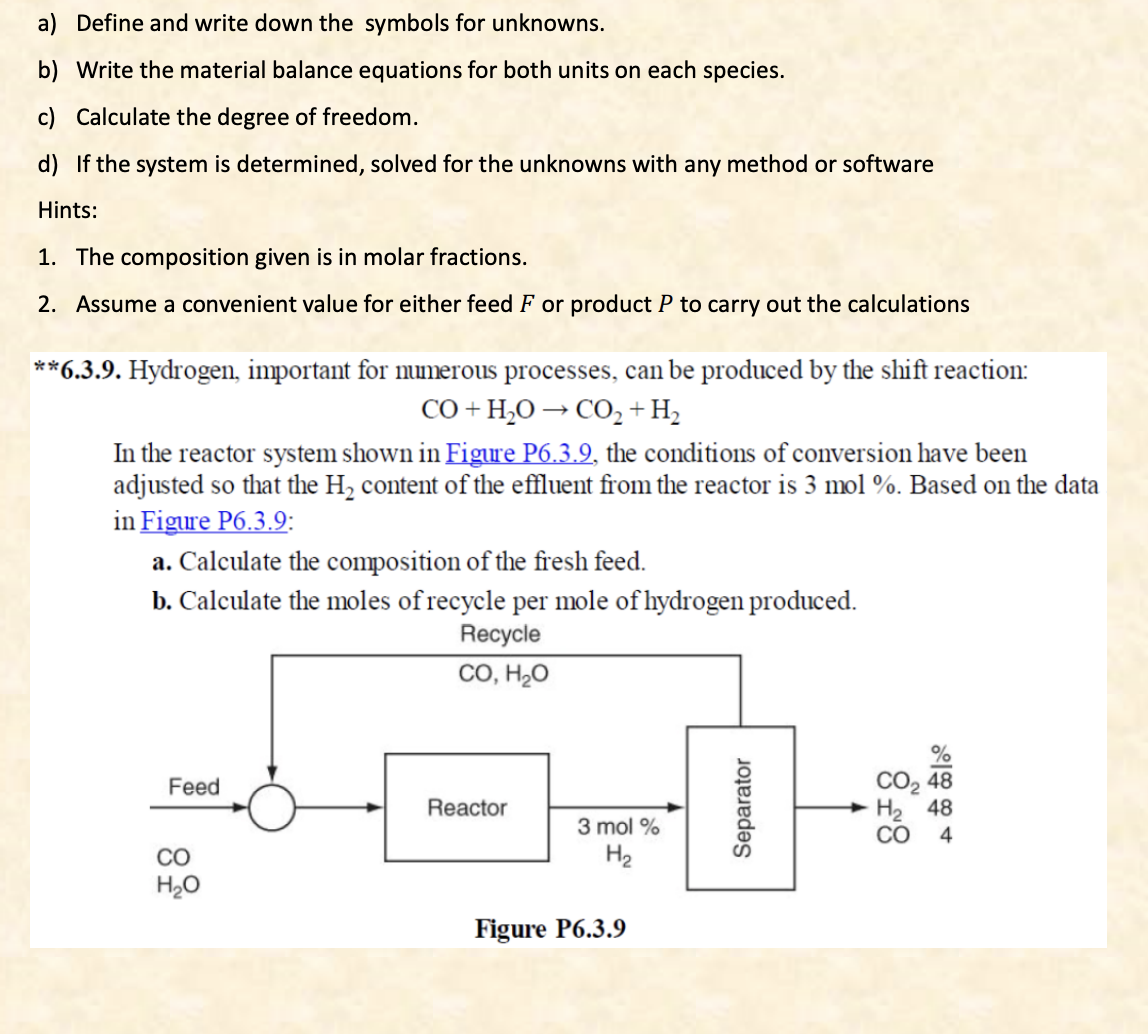
a) Define and write down the symbols for unknowns. b) Write the material balance equations for both units on each species. c) Calculate the degree of freedom. d) If the system is determined, solved for the unknowns with any method or software Hints: 1. The composition given is in molar fractions. 2. Assume a convenient value for either feed F or product P to carry out the calculations **6.3.9. Hydrogen, important for numerous processes, can be produced by the shift reaction: CO+H2OCO2+H2 In the reactor system shown in Figure P6.3.9, the conditions of conversion have been adjusted so that the H2 content of the effluent from the reactor is 3mol%. Based on the data in Figure P6.3.9: a. Calculate the composition of the fresh feed. b. Calculate the moles of recycle per mole of hydrogen produced. a) Define and write down the symbols for unknowns. b) Write the material balance equations for both units on each species. c) Calculate the degree of freedom. d) If the system is determined, solved for the unknowns with any method or software Hints: 1. The composition given is in molar fractions. 2. Assume a convenient value for either feed F or product P to carry out the calculations **6.3.9. Hydrogen, important for numerous processes, can be produced by the shift reaction: CO+H2OCO2+H2 In the reactor system shown in Figure P6.3.9, the conditions of conversion have been adjusted so that the H2 content of the effluent from the reactor is 3mol%. Based on the data in Figure P6.3.9: a. Calculate the composition of the fresh feed. b. Calculate the moles of recycle per mole of hydrogen producedStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


