Question
You should prepare buffer of pH 5.0 of optimal buffer capacity. Which buffer system do you use: a) CH3COONA and CH3COOH, pKA = 4.74;
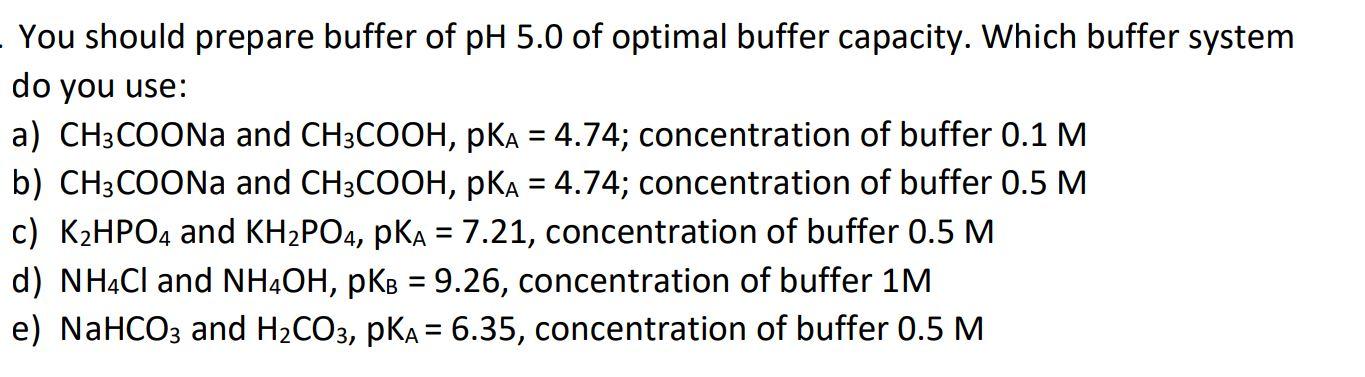
You should prepare buffer of pH 5.0 of optimal buffer capacity. Which buffer system do you use: a) CH3COONA and CH3COOH, pKA = 4.74; concentration of buffer 0.1 M b) CH3COONA and CH3COOH, pKA = 4.74; concentration of buffer 0.5 M c) K2HPO4 and KH2PO4, pKA = 7.21, concentration of buffer 0.5 M %3D %3D %| d) NHCI and NHOH, pKB = 9.26, concentration of buffer 1M e) NaHCO3 and H2CO3, pKA = 6.35, concentration of buffer 0.5 M %3D
Step by Step Solution
3.34 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
The buffer capacity basically depends on two factors 1 Ratio of the salt to the acidbase The ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



