Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You want to make 2.00 liters of a pH buffer at 7.40 (at 25C) using sodium dihydrogen phosphate (NaH 2 PO 4 ; FW 120.0
You want to make 2.00 liters of a pH buffer at 7.40 (at 25C) using sodium dihydrogen phosphate (NaH2PO4; FW 120.0 g/mol) and disodium hydrogen phosphate (Na2HPO4; FW 142.0 g/mol) with a final concentration of total phosphate (NaH2PO4 + Na2HPO4) equal to 24.0 mM.
a. What are the final concentrations of NaH2PO4 and Na2HPO4 (individually) needed to satisfy these conditions? Use the appropriate pK value from Table 2-4.
b. What masses of NaH2PO4 and Na2HPO4 would you use to make this pH buffer? Give your answers to the nearest 0.1 g.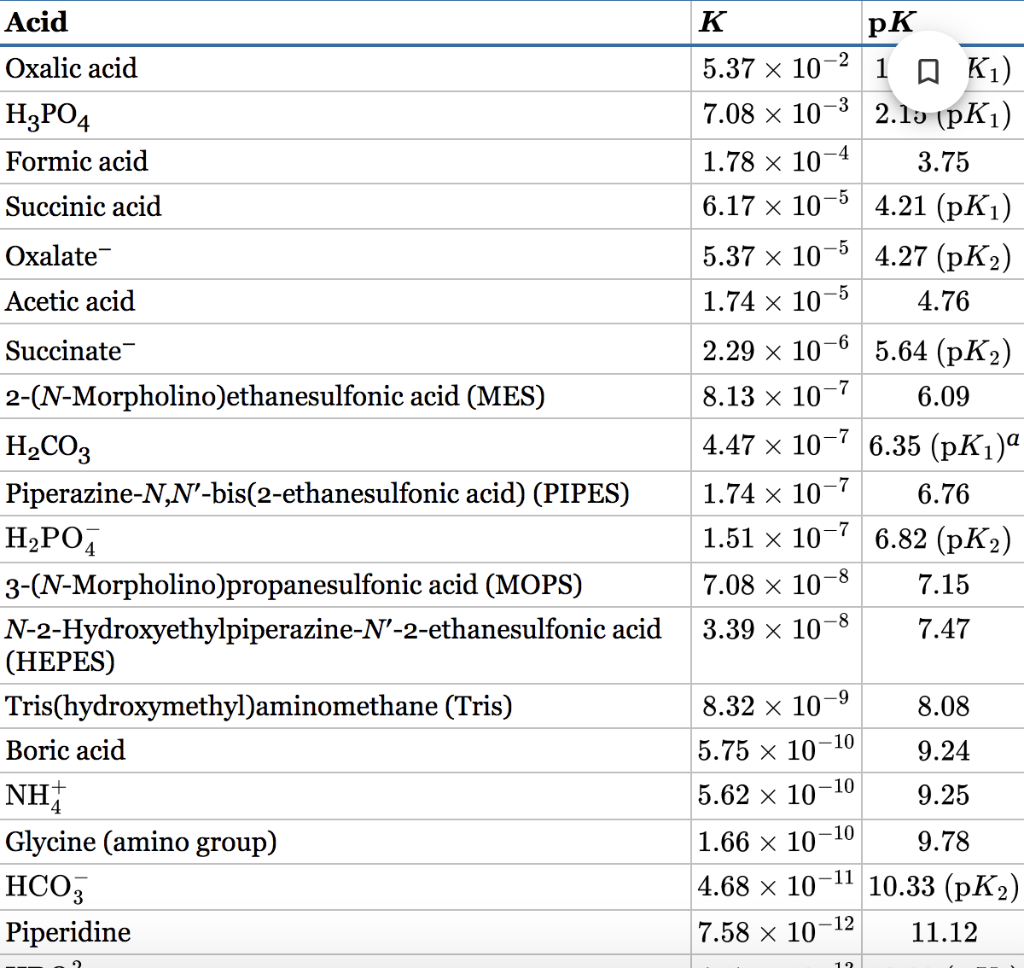
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


