Question
Your lab assignment presentation involves putting together a presentation to show other students on how to perform titration. In other words, your presentation can be
Your lab assignment presentation involves putting together a presentation to show other students on how to perform titration. In other words, your presentation can be used to show other students (next term) how they can perform a titration by following your instructions and calculations. To do this presentation, we are giving you the type of acid, acetic acid, the type of base, NaOH to be used, and you will use phenolphthalein as the indicator. You will use the data given to you to be used as an example of how to solve the mathematical calculations. At the end of your presentation, you will show the unknown concentration of the acetic acid (Molarity) and will also show the % mass/ vol of the Vinegar solution. Part 1 1. Purpose of a titration, what is titration and why do we perform a titration? 2. Instrumentation- what are the instruments, glassware used when performing a titration. 3. Chemicals and Procedures. What chemicals are needed? List and explain the procedures for this experiment. 4. Observations and expectations- List the observations and write your expectations. This might include color change and when the equivalence point (endpoint) might be reached. Part 2 1. Calculations and Results. Show all your calculations and write your results, the Molarity of the acetic and the % of the acetic acid. 2. Your instructor will provide guidance and examples for completing calculations for titration. This assignment should be in current APA Style with both a title slide and a reference list that includes all the sources used. At least two scholarly sources should be used (your textbook can be one of the sources).
Data for Signature assignment: Molarity of NaOH 0.10 M Vol of acetic acid 5.0 mL Trial 1 Trial 2 Trial 3 Initial Burette Reading (NaOH) 0.00 mL 0.00 mL 0.00 mL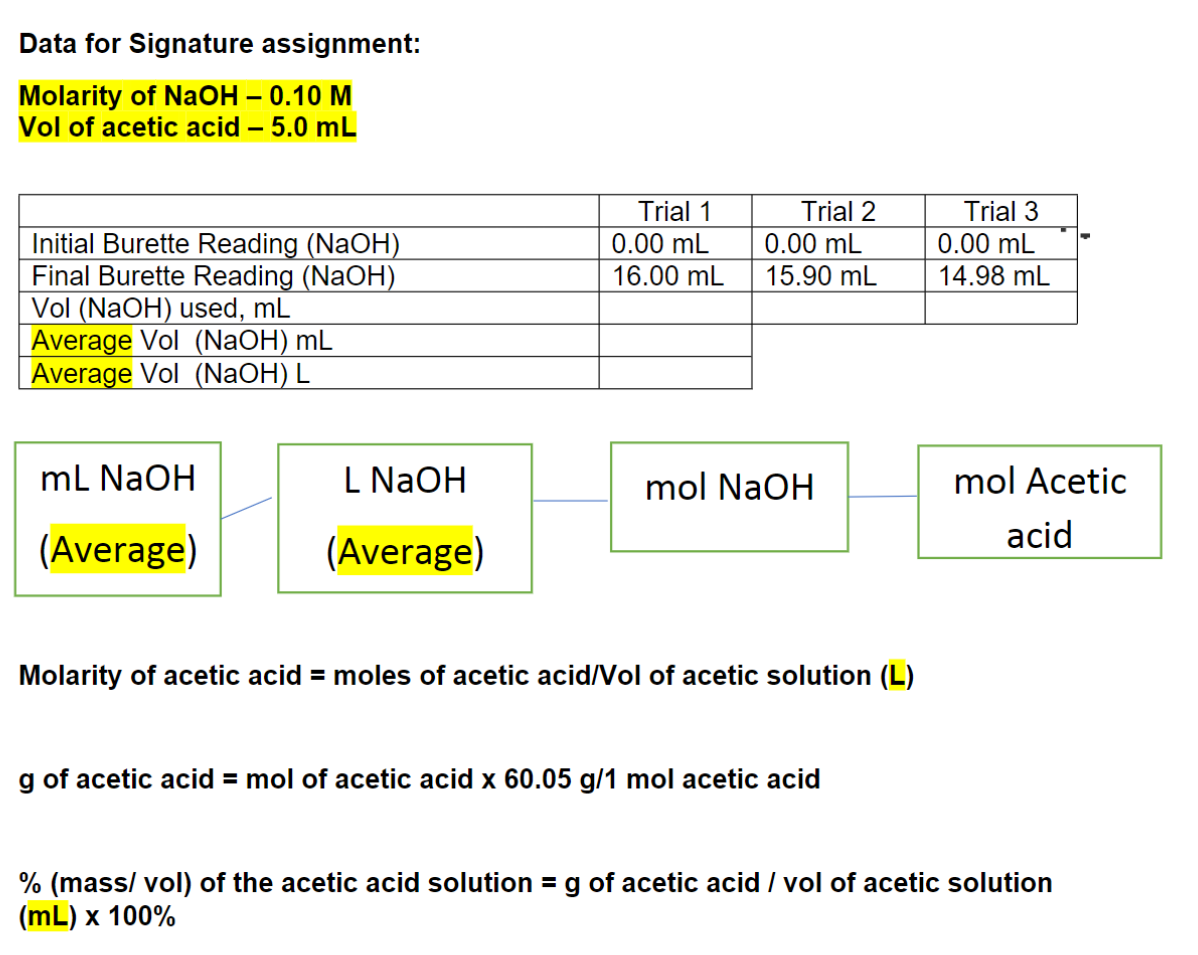 Final Burette Reading (NaOH) 16.00 mL 15.90 mL 14.98 mL Vol (NaOH) used, mL Average Vol (NaOH) mL Average Vol (NaOH) L Molarity of acetic acid = moles of acetic acid/Vol of acetic solution (L) g of acetic acid = mol of acetic acid x 60.05 g/1 mol acetic acid % (mass/ vol) of the acetic acid solution = g of acetic acid / vol of acetic solution (mL) x 100%
Final Burette Reading (NaOH) 16.00 mL 15.90 mL 14.98 mL Vol (NaOH) used, mL Average Vol (NaOH) mL Average Vol (NaOH) L Molarity of acetic acid = moles of acetic acid/Vol of acetic solution (L) g of acetic acid = mol of acetic acid x 60.05 g/1 mol acetic acid % (mass/ vol) of the acetic acid solution = g of acetic acid / vol of acetic solution (mL) x 100%
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


