Question: GaAs (E g = 1.4 eV) and ZnSe (E g = 2.6 eV) are isoelectronic with Ge. Their band structures are shown below. Are they
GaAs (Eg = 1.4 eV) and ZnSe (Eg = 2.6 eV) are isoelectronic with Ge. Their band structures are shown below. Are they direct- or indirect-gap semiconductors?
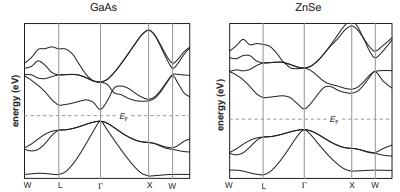
energy (ev) W L GaAs r X W energy (eV) W ZnSe T X W
Step by Step Solution
3.42 Rating (171 Votes )
There are 3 Steps involved in it
For each compound the vale... View full answer

Get step-by-step solutions from verified subject matter experts


