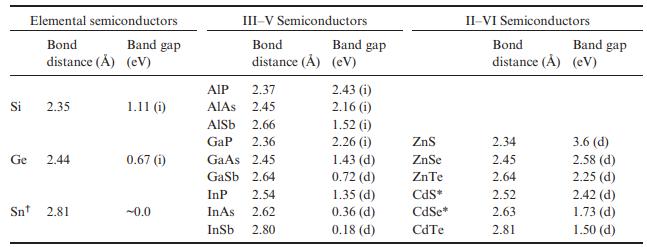
Which semiconductors in Table 7.8 will have colors other than white or black? For each compound in
Question:
Which semiconductors in Table 7.8 will have colors other than white or black? For each compound in your list predict the approximate color.
Table 7.8
Transcribed Image Text:
Si Ge Elemental semiconductors Bond Band gap distance () (eV) 2.35 2.44 Sn 2.81 1.11 (i) 0.67 (1) -0.0 III-V Semiconductors Bond distance () (eV) AIP 2.37 AIAS 2.45 AlSb 2.66 GaP 2.36 2.45 GaAs Gasb InP InAs InSb 2.64 2.54 2.62 2.80 Band gap 2.43 (i) 2.16 (i) 1.52 (i) 2.26 (1) 1.43 (d) 0.72 (d) 1.35 (d) 0.36 (d) 0.18 (d) ZnS ZnSe ZnTe CdS* CdSe* CdTe II-VI Semiconductors Bond distance (A) (eV) 2.34 2.45 2.64 Band gap 2.52 2.63 2.81 3.6 (d) 2.58 (d) 2.25 (d) 2.42 (d) 1.73 (d) 1.50 (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Semiconductors with E g 31 eV do not absorb visible light and will be whi...View the full answer

Answered By

Swati Mann
It was wonderful experience teaching college students .i learnt different ways to deal with all sorts of students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
This case was written by Professor Michele Greenwald, Visiting Professor of Marketing at HEC Paris, for use with Advertising and Promotion: An Integrated Marketing Communications Perspective 7th...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Two protons are separated by a distance of 4.19 cm. Determine the magnitude of the acceleration on the proton. (e=1.602x10-19 C, k-8.99x109 N-m/C, mp-1.672x10-27 kg)
-
The tune - up specifications of a car call for the spark plugs to be tightened to a torque of 3 4 N ?? m . You plan to tighten the plugs by pulling on the end of a 2 8 - cm - long wrench. Because of...
-
A couple of magnitude 50lb ft is applied to the drum. Determine the smallest force that must be exerted by the hydraulic cylinder if the drum is not to rotate when the applied couple is directed (a)...
-
Suppose that, in an attempt to induce citizens to conserve energy, the government enacted regulations requiring that all air conditioners be more efficient in their use of electricity. After this...
-
An unmanned monitoring system uses high-tech video equipment and microprocessors to detect intruders. A prototype system has been developed and is in use outdoors at a weapons munitions plant. The...
-
The volume of a dry box (a closed chamber with dry nitrogen flowing through it) is 2.0 m3. The dry box is maintained at a slight positive gauge pressure of 10 cm H2O and room temperature (25C). If...
-
On June 1, 2018, Dirty Harry Co. borrowed cash by issuing a 6-month noninterest-bearing note with a maturity value of $420,000 and a discount rate of 9%. Assuming straight-line amortization of the...
-
The red pigment vermillion (HgS) is a semiconductor with a band gap of 2.0 eV. It was replaced in the late nineteenth and early twentieth century by CdS 1x Se x pigments that are less toxic and more...
-
Tetrathiomolybdate MoS 4 2 is tetrahedral and features a strong t1 e LMCT absorption at 470 nm. (a) What color would you predict for (NH 4 ) 2 MoS 4 ? (b) What is the energy in eV of the LMCT...
-
What mass of FeCl2 is present in 445 mL of 0.0812 M FeCl2 solution?
-
During the COVID-19 19 pandemic, 50 patients over the age of 60 were admitted to a hospital, where 25 patients were completely cured without needing to enter the intensive care rooms. The ages of 25...
-
In Exercises 5-20, find the range, variance, and standard deviation for the given sample data. Include appropriate units (such as "minutes") in your results. (The same data were used in Section 3-1,...
-
00 0.1 11 points eBook Smoky Mountain Corporation makes two types of hiking boots-the Xtreme and the Pathfinder. Data concerning these two product lines appear below: Selling price per unit Xtreme...
-
Computing Unit Cost Comacho Chemical Co. recorded costs for the month of $18,900 for materials, $44,100 for labor, and $26.250 for factory overhead. There was no beginning work in process, 8.000...
-
Problem Joe Fox is the manager of the Gear division and plans to submit a proposal for an expanded production area. Joe has projected various conditions for revenues and expense for the expansion,...
-
Air Borealis works only with advance reservations and experiences a 7% rate of no-shows. How many reservations could be accepted for an airliner with a capacity of 250 if there is at least a 0.95...
-
A copper rod of length L =18.0 in is to be twisted by torques T (see figure) until the angle of rotation between the ends of the rod is 3.08. (a) If the allowable shear strain in the copper is 0.0006...
-
Compound A has molecular formula C 5 H 10 . Hydroboration- oxidation of compound A produces 2-methylbutan-1-ol. Draw the structure of compound A: Compound A (C,H10) 1) BH, THF 2) H202, NaOH
-
Explain how the ideal gas law can be deduced for the measurements shown in Figures 1.5 and 1.8. Figure 1.5 Figure 1.8 0.1 L 2.5 2.0 1.5 0.2 L 1.0 0.3 L 0.4 L 0.5 L 0.6 L '0.5 -200 100 0 100 200 300...
-
A bowling ball (a) Rolls across a table (b) Falls on the floor. Is the work associated with each part of this process positive, negative, or zero?
-
Columbus Industries makes a product that sells for $37 a unit. The product has a $29 per unit variable cost and total fixed costs of $10,000. At budgeted sales of 1,950 units, the margin of safety...
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year

Study smarter with the SolutionInn App


