Question: In its charged state, a leadacid battery contains PbO 2 and Pb electrodes. The half equations that occur during discharge can be written as below.
In its charged state, a lead–acid battery contains PbO2 and Pb electrodes. The half equations that occur during discharge can be written as below. Give the overall cell equation and estimate E° cell. State which electrode is the cathode and which the anode.
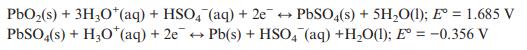
PbO(s) + 3HO*(aq) + HSO4 (aq) + 2e + PbSO4(s) + 5HO(1); E = 1.685 V PbSO4(s) + HO (aq) + 2e Pb(s) + HSO4 (aq) +HO(1); E = -0.356 V
Step by Step Solution
★★★★★
3.47 Rating (157 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The overall reaction and Ecell can be determined by subtracting ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


