Question: Write out half equations, the cell reaction, and determine the standard potential of the following cells given standard electrode potentials E(Cu+/Cu) = +0.34 V, E(Sn*/Sn)
Write out half equations, the cell reaction, and determine the standard potential of the following cells given standard electrode potentials
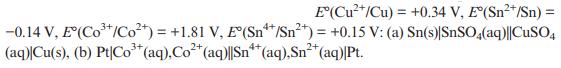
E(Cu+/Cu) = +0.34 V, E(Sn*/Sn) = -0.14 V, E(Co*/Co) = +1.81 V, E(Sn/Sn+) = +0.15 V: (a) Sn(s)|SnSO4(aq)||CuSO4 3+ 2+ (aq)|Cu(s), (b) Pt|Co+ (aq), Co+ (aq)|Sn** (aq), Sn+ (aq)|Pt.
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
a Cu 2 2e Cu E 034 V higher standard potential defines the oxidizing agent Sn 2 2e ... View full answer

Get step-by-step solutions from verified subject matter experts


