Question: Write down the half equations that occur at each electrode in the oxygen sensor of Figure 13.17 and state the overall cell equation. Oxygen sensors
Write down the half equations that occur at each electrode in the oxygen sensor of Figure 13.17 and state the overall cell equation. Oxygen sensors of this type are used in car engines to monitor the air to fuel ratio via changes in the partial pressure of O2 in the exhaust gas relative to air. Calculate the voltage expected from the sensor for the following air to fuel (A:F) ratios:
(a) A lean mixture of A:F = 10.5 and p(O2) = 1×10−14 Pa,
(b) A stoichiometric ratio of A:F = 14.7 and p(O2) = 5×10−11 Pa,
(c) A rich mixture of A:F = 18 and p(O2) = 4500 Pa. Assume the partial pressure of O2 in air is 21.2 kPa and the exhaust gases are at 1000 K.
Figure 13.17
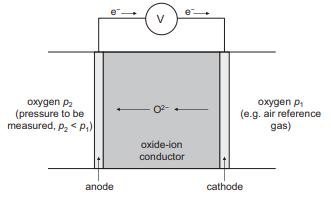
oxygen P (pressure to be measured, p
Step by Step Solution
3.49 Rating (175 Votes )
There are 3 Steps involved in it
Righthand electrode O 2 p 1 4e 2O 2 Lefthand O 2 p 2 4e 2O ... View full answer

Get step-by-step solutions from verified subject matter experts


