Suggest oxidation states for the metal ions in each of the following materials: (a) TiS2, Lio.7 TiS2;
Question:
Suggest oxidation states for the metal ions in each of the following materials:
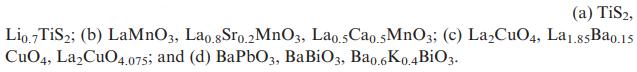
Transcribed Image Text:
(a) TiS2, Lio.7 TiS2; (b) LaMnO3, Lao.8Sro.2 MnO3, Lao.5Cao.5MnO3; (c) LaCuO4, La1.85Ba0.15 CuO4, LaCuO4.075; and (d) BaPbO3, BaBiO3, Ba0.6K0.4BiO3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Oxidation states a Ti4 note difference from FeS2 in previous Problem Li Ti ...View the full answer

Answered By

Usman Nasir
I did Master of Commerce in year 2009 and completed ACCA (Association of Chartered Certified Accountants) in year 2013. I have 10 years of practical experience inclusive of teaching and industry. Currently i am working in a multinational company as finance manager and serving as part time teacher in a university. I have been doing tutoring via many sites. I am very strong at solving numerical / theoretical scenario-based questions.
4.60+
16+ Reviews
28+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Suggest oxidation states for the metal ions in each of the following materials: (a) FeO, Feo.8720, Fe3O4, FeS2; (b) FeTe, Fe1 Te; (c) LaOFeAs, LaO0.9Fo.1 FeAs; and (d) YBaFeO5, NdBaFeO5.5, and...
-
What strategy would be the most useful to companies interested in Brazilian investment?
-
State the predicted ionic charge of metal ions in each of the following groups of elements. (a) Group IA/1 (b) Group IIA/2 (c) Group IIIA/13 (d) Group IVA/14.
-
a. Distinguish between internal common equity and new common stock. b. Why is there a cost associated with internal common equity? c. Describe two approaches that could be used in computing the cost...
-
Derive an expression for the magnitude of the couple M required to maintain the equilibrium of the linkage shown.
-
What are three goals of inventory management?
-
2. Are additional reportable segments required?
-
The physical distribution channel of a major food manufacturer consists of plant stocks from which regional warehouses are restocked. These regional warehouses in turn supply the field warehouses...
-
Kathy Myers frequently purchases stocks and bonds, but she is uncertain how to determine the rate of return that she is earning. For example, three years ago she paid $19,000 for 930 shares of Malti...
-
A brown sample of zinc oxide was found to have the hexagonal wurtzite structure with a = b = 3.2495 , c = 5.2069 ( = = 90; = 120). Chemical analysis gave 80.765% Zn by mass. Density measurements...
-
TiO with a 1:1 ratio of Ti:O was synthesized and found to have a NaCl-related structure with a = 4.1831 and an experimental density of 4927 kg/m 3 . Comment on these values.
-
The Nissan Leaf is an all-electric car powered by a 107-hp electric motor and a lithium-ion battery that stores 24 kWh and produces 394 V at its terminals when fully charged. The Leafs battery can...
-
n1 = 15, n2 = 18, S = 280, H1: m1 > m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
n1 = 25, n2 = 32, S = 850, H1: m1 m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
Evaluate the matrix element $\left\langle j_{1} j_{2} J\left|T_{k q}(1) ight| j_{1}^{\prime} j_{2}^{\prime} J^{\prime} ightangle$, where the tensor operator $T_{k q}(1)$ operates only on the part of...
-
Mark Gold opened Gold Roofing Service on April 1. Transactions for April are as follows: 1 Gold contributed \(\$ 15,000\) of his personal funds in exchange for common stock to begin the business. 2...
-
n1 = 20, n2 = 30, S = 400, H1: m1 < m2. Exercises 57 present sample sizes and the sum of ranks for the rank-sum test. Compute S, S, and the value of the test statistic z. Then find the P-value.
-
Leadership acts occur when one person tries to influence the behavior of others toward the attainment of some goal. The effects of leadership style on the behavior of subordinates were investigated...
-
Consider the combustion of methanol below. If 64 grams of methanol reacts with 160 grams of oxygen, what is the CHANGE in volume at STP. 2CH3OH(g) + 3O2(g) 2CO2(g) + 4H2O(1) The volume decreases by...
-
Propose a mechanism for each of the following reactions: a. b. c. d. ,* ,*
-
Identify reagents that can be used to achieve each of the following transformations: a. b. c. d. e. f. Br Br HO,
-
Draw a Lewis structure for each of the following ions; in each case, indicate which atom possesses the formal charge: a. BH 4 b. NH 2 c. C 2 H 5 +
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...

Study smarter with the SolutionInn App


