Suggest oxidation states for the metal ions in each of the following materials: (a) FeO, Feo.8720, Fe3O4,
Question:
Suggest oxidation states for the metal ions in each of the following materials:
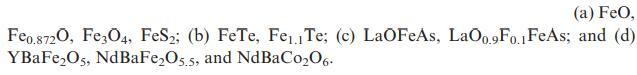
Transcribed Image Text:
(a) FeO, Feo.8720, Fe3O4, FeS2; (b) FeTe, Fe1 Te; (c) LaOFeAs, LaO0.9Fo.1 FeAs; and (d) YBaFeO5, NdBaFeO5.5, and NdBaC006.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Oxidation states a Fe Fe29 or Fe 0616Fe0256 Fe266 of 1 Fell per 2 Fell in a spinel Fe...View the full answer

Answered By

Hillary Waliaulah
As a tutor, I am that experienced with over 5 years. With this, I am capable of handling a variety of subjects.
5.00+
17+ Reviews
30+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Suggest oxidation states for the metal ions in each of the following materials: (a) TiS2, Lio.7 TiS2; (b) LaMnO3, Lao.8Sro.2 MnO3, Lao.5Cao.5MnO3; (c) LaCuO4, La1.85Ba0.15 CuO4, LaCuO4.075; and (d)...
-
What strategy would be the most useful to companies interested in Brazilian investment?
-
State the predicted ionic charge of metal ions in each of the following groups of elements. (a) Group IA/1 (b) Group IIA/2 (c) Group IIIA/13 (d) Group IVA/14.
-
The following list of balances has been extracted from the records of company cowgale co as of 31 October 2017 the end of the most recent financial year. Notes 1. The balance on the corporation tax...
-
Derive an expression for the magnitude of the couple M required to maintain the equilibrium of the linkage shown. M.
-
Use the information in PB6-1 to complete the following requirements. Info PB6-1 a. Southern Sporting Goods sold merchandise to Sports R Us at a selling price of $ 125,000. The merchandise had cost...
-
5. In January 2016, Pin Company paid property taxes of $80,000 covering the calendar year 2016. Also in January 2016, Pin estimated that its year-end bonuses to executives would amount to $320,000...
-
Andys Autobody Shop has the following balances at the beginning of September: Cash, $10,000; Accounts Receivable, $1,450; Equipment, $40,000; Accounts Payable, $2,000; Common Stock, $20,000; and...
-
Op managers of Preston Industries predicted 2018 sales of 15,100 units of its product at a unit price of $7.00. Actual sales for the year were 14,800 units at $11.50 each. Variable costs were...
-
A sample of nonstoichiometric nickel oxide (A) was found to contain 77.70% Ni by mass. (a) Calculate the empirical formula of A and state the two alternatives for the intrinsic defect that would on...
-
A brown sample of zinc oxide was found to have the hexagonal wurtzite structure with a = b = 3.2495 , c = 5.2069 ( = = 90; = 120). Chemical analysis gave 80.765% Zn by mass. Density measurements...
-
True or False sin( + ) = sin + sin +2 sin sin
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
A coagulation-microfiltration process for removing bacteria from water was investigated in Environmental Science & Engineering (Sept. 1, 2000). Chemical engineers at Seoul National University...
-
The following items were displayed in the statement of affairs for Lubbock Company: Fully secured liabilities ......... $90,000 Partially secured liabilities ....... 12,000 Unsecured liabilities...
-
There are four constitutional isomers with molecular formula C 3 H 9 N. Draw a Lewis structure for each isomer and determine the number of lone pairs on the nitrogen atom in each case?
-
Would water be a suitable proton source to protonate the following compound? ONa
-
Predict the major product(s) for each of the following reactions: 1) MCPBA 1) , THF 2),, NaOH 2) ,* z . Pt
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


