Question: The figure below shows E against x at 30 C for a cell with a spinel-based LixMn 2 O 4 cathode (0 (a) Describe the
The figure below shows E against x at 30 °C for a cell with a spinel-based LixMn2O4 cathode (0
(a) Describe the structure you might expect for a LiMn2O4 spinel.
(b) State what can you conclude from Ecell versus x in the regions 0
(c) State what could cause the marked drop in Ecell at x = 1.
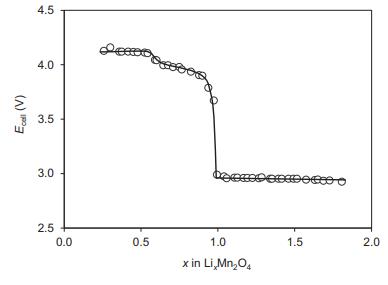
Ecal (V) = 4.5 4.0 3.5 3.0 2.5 0.0 0.5 bet 1.0 x in Li,Mn>O4 1.5 2.0
Step by Step Solution
3.31 Rating (151 Votes )
There are 3 Steps involved in it
The electrochemical data are taken from Ohzuku et al J Electrochem Soc 137 1990 769775 a Spinel has ... View full answer

Get step-by-step solutions from verified subject matter experts


