Use the MO diagram of oxygen to determine the oxygenoxygen bond order in the peroxide ion, O
Question:
Use the MO diagram of oxygen to determine the oxygen–oxygen bond order in the peroxide ion, O22 . Will the O–O distance in peroxide be longer or shorter than in O2?
MO diagram
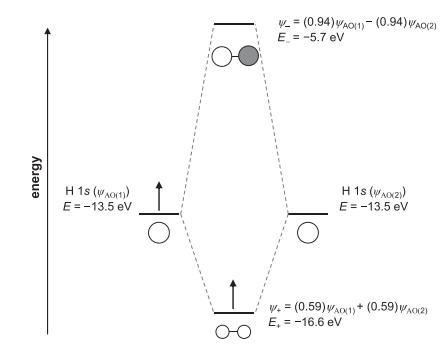
Transcribed Image Text:
energy H 1s ((1)) E = -13.5 eV Ho _= (0.94) WAO(1)- (0.94) WAO(2) E = -5.7 eV To H 1S (WA(Z)) E = -13.5 eV W+= (0.59) WAO(1) + (0.59) A0(2) E, = -16.6 eV
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Peroxide has two more electrons than O 2 Ther...View the full answer

Answered By

Ashish Bhalla
I have 12 years work experience as Professor for Accounting, Finance and Business related subjects also working as Online Tutor from last 8 years with highly decentralized organizations. I had obtained a B.Com, M.Com, MBA (Finance & Marketing). My research interest areas are Banking Problem & Investment Management. I am highly articulate and effective communicator with excellent team-building and interpersonal skills; work well with individuals at all levels.
4.80+
17+ Reviews
46+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
The oxygenoxygen bond in O2+ is 112 pm and in O2 is 121 pm. Explain why the bond length in O2+ is shorter than in O2. Would you expect the bond length in O2 to be longer or shorter than that in O2?...
-
Use the molecular orbital diagram in Fig. 17.5 to determine the bond order of the Br 2 + ion. Will the BrBr bond be longer or shorter than that in the Br 2 molecule? Figure 17.5. Energy 3s 20 1 1...
-
(a) Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves an octet of electrons. (b) Do you expect the O-O bond in H2O2 to be longer or shorter than the bond in O2?
-
The input file for this assignment is Weekly_Gas_Average.txt. The file contains the average gas price for each week of the year. Write a program that reads the gas prices from the file into an...
-
Determine by direct integration the product of inertia of the given area with respect to the x and y axes.
-
Given C = 100 + 0.8Y, M = 150 + 0.20Y, I = 100, and X = 350: (a) Determine YE algebraically. (b) Show the determination of YE graphically as in the top panel of Figure 17.3.
-
1.17 .331 Females
-
Where would each of the following items most likely be reported in a companys financial statements? Assume the monetary amount of each item is material. 1. Bad debts expense 2. Sales discounts taken...
-
Minden Company is a wholesale distributor of premium European chocolates. The companys balance sheet as of April 30 is given below: Minden Company Balance Sheet April 30 Assets Cash $ 9,000 Accounts...
-
Construct an MO diagram for trigonal-planar BH3 by analogy with the MOs for trigonal-planar NH 3 in Figure 5.26. Use this diagram to determine the degeneracy and orbital character of the HOMO and the...
-
What are the values of the principal and orbital angular-momentum quantum numbers for each of the following orbitals? How many radial nodes and nodal planes does each orbital possess? (a) 4s orbital,...
-
The following information was taken from the balance sheet of Cohort Enterprises as of December 31, 2018: The bonds have a stated interest rate of 5 percent paid annually and will mature on December...
-
Alvarado Company produces a product that requires 5 standard direct labor hours per unit at a standard hourly rate of $12.00 per hour. If 5,700 units used 29,400 hours at an hourly rate of $11.40 per...
-
7. (30 points) You are a teaching assistant (TA) for a new course in the department and you wish to measure the amount of time that students spend engaging with the online resources. Using the Canvas...
-
Mod Clothiers makes women's clothes. It costs $28,000 to produce 5,000 pairs of polka-dot polyester pants. They have been unable to sell the pants at their usual price of $50.00. The company is...
-
In a mid-sized manufacturing company, the annual financial statements were prepared for audit by an external auditing firm. The company\'s finance team had diligently compiled the financial data, and...
-
Explain the meaning of the SMART acronym. In 100-200 words, define what the words "goal" and "success" mean to you. Summarize your thoughts on whether or not the SMART model can help you become a...
-
The skid properties of a snow tire have been tested, and a mean skid distance of 47 m has been established for standardized conditions. A new, more expensive tire is developed, but tests on a sample...
-
Find the APR in each of the following cases: NUMBER OF TIMES COMPOUNDED Semiannually Monthly Weekly Infinite EAR APR 10.4% 8.9 11.6 15.4
-
Using acetylene and methyl bromide as your only sources of carbon atoms, propose a synthesis for each of the following compounds: (a) (b) En Et Me En Et Me
-
Draw a mechanism and predict the major product for each reaction. a. b. c. d. e. f. ? 1) LAH H. 2) H20
-
Propose a molecular formula for a compound that exhibits the following peaks in its mass spectrum. a) (M) + at m/z = 72, relative height = 38.3% of base peak (M+1) + at m/z = 73, relative height =...
-
Present Value Computations Using the present value tables, solve the following. ( Click here to access the PV and FV tables to use with this problem. ) Round your answers to two decimal places....
-
A company provided the following data: Sales $887,000 Variable costs $546,800 Fixed costs $310,000 Expected production and sales in units 36,000 What is the break-even point in sales dollars? Please...
-
How to solve them..equation and explain ..please.. 1. Selected information from the companys financial records is presented below Equipment, December 31, 2013 $300,000 Equipment, December 31, 2014...

Study smarter with the SolutionInn App


