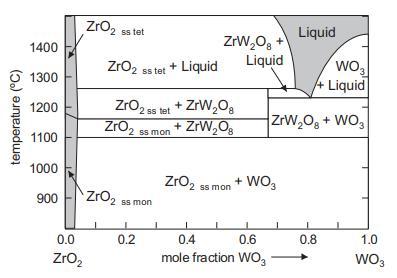
Question: Using the phase diagram of Figure 4.8: (a) State how you would attempt to prepare a solid polycrystalline sample of ZrW 2 O 8 .
Using the phase diagram of Figure 4.8:
(a) State how you would attempt to prepare a solid polycrystalline sample of ZrW2O8.
(b) State how you would attempt to grow single crystals of ZrW2O8.
Figure 4.8
temperature (C) 1400 1300 1200 1100 1000 900 0.0 ZrO ZrO ss tet ZrO2 ss tet + Liquid ZrO2 ss tet 2 ss tet + ZrW08 ZrO2 ss mon + ZrW Og ZrO 2 ss mon ZrW08 0.2 Liquid ZrO ss mon + WO3 0.6 0.4 mole fraction WO3 Liquid WO3 + Liquid ZrW08 + WO3 0.8 1.0 WO3
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
From Figure 48 ZrW 2 O 8 is thermodynamically stable between 1105 and 1257 C One can therefore sinte... View full answer

Get step-by-step solutions from verified subject matter experts


