Show that the reciprocal lattice of a simple hexagonal lattice (see Table 1.1) is also a simple
Question:
Show that the reciprocal lattice of a simple hexagonal lattice (see Table 1.1) is also a simple hexagonal, with lattice constants 2π/c and 4π/√3a, rotated through 30° about the c-axis with respect to the real-space lattice.
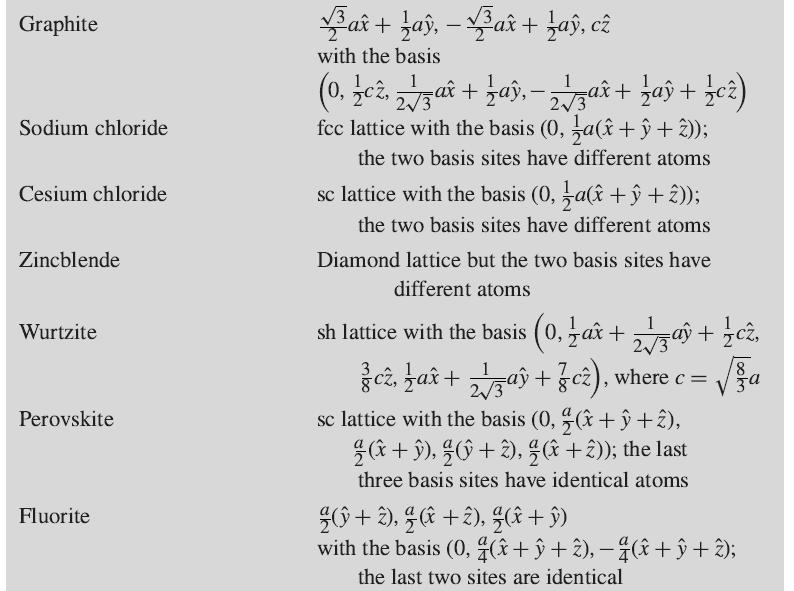

Transcribed Image Text:
Table 1.1 Common crystal structures Structure Simple cubic (sc) Body-centered cubic (bcc) Face-centered cubic (fcc) Standard primitive vectors and basis ax, ay, az ax, ay, a(x + y + 2) or sc lattice with the basis (0, a( +ŷ + 2)) {a(ŷ + 2), ¼a(2+x), ¼a(x +ŷ) or sc lattice with the basis (0, ½a(î + ŷ), ⁄a(ŷ + 2), ½a(2 + x)) Diamond fcc lattice with the basis (0, a(+ŷ + 2)) ax, (¹ax +ay), c² Simple hexagonal (sh) Hexagonal close-packed (hcp) sh lattice with the basis (0, -ax+ 2√3aŷ + ½cz), where c = 8 V za
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
In the simple hexagonal lattice the basis vectors are ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) A graphene lattice, or honeycomb lattice, is the same as the graphite lattice (see Table 1.1) but consists of only a two-dimensional sheet with lattice vectors and a two-atom basis including only...
-
As a follow-up to Exercise 1.4.6, show that the special point at which the gap energy goes to zero in (1.9.19) for the graphene lattice is one of the corners of the WignerSeitz cell for the Brillouin...
-
Hexagonal emit-packed structure Consider first Brillouin zone of a crystal with a simple hexagonal lattice in three dimensions with lattice constants a and c. Let G c denote the shortest reciprocal...
-
On January 2, 2013, Parker Corporation invests in the stock of Quarry Corporation. Quarry's book value is $4 million and its assets and liabilities are fairly reported. Quarry reports income of $3...
-
LaPlatt & Associates is an accounting rm that provides audit, tax, and accounting services to medium-size retail companies. It employs 50 professionals (10 partners and 40 associates) who work...
-
Visit https://www.pearsonglobaleditions.com/Horngren to view a link to Target Corporations 2018 Fiscal Year Annual Report. Study the audit opinion (labeled Report of Independent Registered Public...
-
How do intercompany debt and related interest affect the consolida tion process in the year of acquisition as well as in succeeding periods? LO4
-
A production manager at a Contour Manufacturing plant has inspected the number of defective plastic molds in 5 random samples of 20 observations each. Following are the number of defective molds...
-
Cane Company manufactures two products called Alpha and Beta that sell for $175 and $135, respectively. Each produc uses only one type of raw material that costs $5 per pound. The company has the...
-
Consider Table 16.13. Verify (calculate) that S 3 , an estimate made in period 3 of 0 , is 359.276. Also verify (calculate) that the one-period-ahead forecast error for period 4 is -62.276, as shown...
-
Use Mathematica to plot Re k as a function of E = h 2 K 2 /2m using Equation 1.2.6. Assume that you have a set of units such that h 2 /2m = 1, set a = 1, and choose various values of U 0 b from 0.1...
-
Again, consider the daily simple returns of GM stock in the file d-gmsp9908.txt. (a) Find an adequate GARCH-M model for the series. Write down the fitted model. (b) Find an adequate EGARCH model for...
-
who do you think sets the underlying ethical standards when the law is fuzzy on an issue? as business and societal issues develop in the future, how does your opinion in this area inform your...
-
how do i introduce low risk high reward for a new medical assistant supervisor role in an organization?
-
How do individual differences in cognitive styles, such as analytical versus intuitive thinking, impact problem-solving approaches and decision-making processes within teams ?
-
In Russian government, do you think that Russian Military Performance is good in warfare against Ukraine? Explain.
-
Why do you think the competing values framework is important to an organization's effectiveness? Describe the four profiles of the competing values framework. Identify one of the profiles and provide...
-
Consider the corrupt culture at Daimler. Why do you think that the practice of making routine bribes persisted for so many years? Why do you think no one blew the whistle on the practice?
-
(a) Use integration by parts to show that (b) If f and g are inverse functions and f' is continuous, prove that (c) In the case where f and t are positive functions and b > a > 0, draw a diagram to...
-
Draw resonance structures for each of the following compounds: a. b. c. d. e. f. g. h. i. j. N.
-
When the following optically active alcohol is treated with HBr, a racemic mixture of alkyl bromides is obtained: Draw the mechanism of the reaction, and explain the stereochemical outcome. Br HBr +...
-
When ethylbenzene is treated with NBS and irradiated with UV light, two stereoisomeric compounds are obtained in equal amounts. Draw the products and explain why they are obtained in equal amounts....
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


