Show the consistency of the exchange energy formula (8.12.15) and the result of integrating (8.12.3). Fock(k) =
Question:
Show the consistency of the exchange energy formula (8.12.15) and the result of integrating (8.12.3).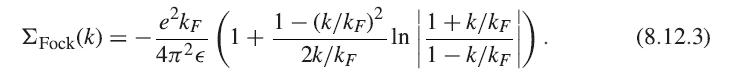

Transcribed Image Text:
ΣFock(k) = - e²kF 4л²€ 1+ 1 - (k/kF)² 2k/kF -In 1+k/kF 1 - k/kF (). (8.12.3)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (12 reviews)
In order to prove the statement we must calculate the exchange ene...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-4. Ivan and Irene paid the following in 2012 (all by check or can otherwise be...
-
Accounts Receivable AED 5,000 Accounts Payable 15,000 Advertising Expense 2,000 16,500 Building Cash Common Stock 50,000 Dividends 1,200 Equipment 2,000 Land 70,000 Notes Payable 60,000 3,400 Office...
-
Tatum Company has four products in its inventory. Information about the December 31, 2011, inventory is as follows: The normal gross profit percentage is 25% of cost. Required: 1. Determine the...
-
Jaffe Company incurs annual fixed costs of \($60,000\). Variable costs for Jaffes product are \($7.50\) per unit, and the sales price is \($12.50\) per unit. Jaffe desires to earn an annual profit of...
-
Leadership: establishing direction, unity of purpose, and a supportive work environment? LO.1
-
Dugan Sales had the following transactions for jackets in 2014, its first year of operations: During the year, Dugan Sales sold 830 jackets for $40 each. Required a. Compute the amount of ending...
-
Granite Company purchased a machine costing $125,000, terms 3/10, n/30. The machine was shipped FOB shipping point and freight charges were $2,500. The machine requires special mounting and wiring...
-
According to (4.8.15) in Section 4.8, the scattering rate for fermions is proportional to 1/2 (U D U E ) 2 , where UD is the direct interaction vertex and U E is the exchange vertex. In the diagram...
-
Show that the Matsubara Greens function is periodic, that is, for prove Use the cyclic property of traces (8.13.10), above. G(T T) = -i(T(az(t)a/(t)))T, k
-
A proton and an electron travel through a region of uniform magnetic field \(\vec{B}\). If their speeds are the same, what is the ratio \(R_{\mathrm{p}} / R_{\mathrm{e}}\) of the radii of their...
-
Q13. The probability that Ryan will roll a three using a standard die is 1/6. Let Y = number of times that Ryan has to roll a die in order to roll the first three. What is the expected value for Y?...
-
1. The following are data for two IT projects for a new database system. Prepare a spreadsheet for two projects, using the following data. Amounts are in thousands of dollars. Calculate the NPV for...
-
The Matsui Lubricants plant uses the weighted-average method to account for its work-in-process inventories. The accounting records show the following information for a particular day: Beginning WIP...
-
James Cook, a production department worker, is paid on hourly basis at a rate of $15 per hour. James works 40 hours per week. Any time James works over 40 hours, it is considered as overtime and he...
-
You just started working as a Health Service Manager within one of the following healthcare industries. First, choose an industry below to discuss the questions that follow: Ambulatory Surgery center...
-
Given that people really do defer to experts, how might the leader establish his or her expertise?
-
State whether each statement is true or false. If false, give a reason. {purple, green, yellow} = {green, pink, yellow}
-
For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic: (a) (b) (c) (d) (e) Discuss. OMe . CI H,
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
Consider the structure of N,N-dimethylformamide (DMF): We might expect the two methyl groups to be equivalent; however, both the proton and carbon NMR spectra of DMF show two separate signals for the...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...
-
The following is part of the computer output from a regression of monthly returns on Waterworks stock against the S&P 5 0 0 index. A hedge fund manager believes that Waterworks is underpriced, with...
-
Doisneau 25-year bonds have an annual coupon interest of 8 percent, make interest payments on a semiannual basis, and have a $1,000 par value. If the bonds are trading with a market's required yield...

Study smarter with the SolutionInn App


