Consider a binary mixture composed of two types of particles, A and B. For this system the
Question:
Consider a binary mixture composed of two types of particles, A and B. For this system the fundamental equation for the Gibbs free energy is G=nH+B, the combined first and second laws are dG-S dT+ V dP+ Adna + Bdng (S is the total entropy and V is the total volume of the system), and the chemical potentials A and g are intensive so that AA(P,T,xA) and B = B(P, T, XA), where x is the mole fraction of A. Use these facts to derive the relations
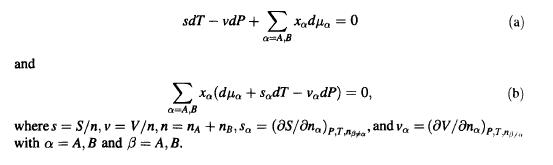
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:






