Consider liquid mixture (!) of particles A and B coexisting in equilibrium with vapor mixture (g) of
Question:
Consider liquid mixture (!) of particles A and B coexisting in equilibrium with vapor mixture (g) of particles A and B. Show that the generalization of the Clausius-Clapeyron equation for the coexistence curve between the liquid and vapor phases when the mole fraction of A in the liquid phase is held fixed is given by
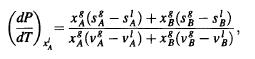
where a = (a/ana) PT and Va = (JV/ana) P.Tata with a = A, B and B = A, B. [Hint: Equation
(b) of Problem (3.8) is useful.]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:






