Problem S2.4. An insulated box with fixed total volume V is partitioned into m insulated compartments, each
Question:
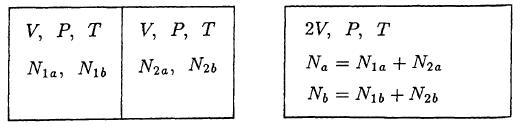
Problem S2.4. An insulated box with fixed total volume V is partitioned into m insulated compartments, each containing an ideal gas of a different molecular species. Assume that each compartment has the same pressure but a different number of moles, a different temperature, and a different volume. (The thermodynamic variables for the ith compartment are (P, ni, Ti, Vi).) If all partitions are suddenly removed and the system is allowed to reach equilibrium:
(a) Find the final temperature and pressure, and the entropy of mixing. (Assume that the particles are monatomic.)
(b) For the special case of m= 2 and parameters n = 1 mol, T = 300 K, V = 1 liter, n = 3 mol, and V = 2 liters, obtain numerical values for all parameters in part (a). =
Step by Step Answer:







