Problem S2.6. A solution of particles A and B has a Gibbs free energy Initially, the solution
Question:
Problem S2.6. A solution of particles A and B has a Gibbs free energy
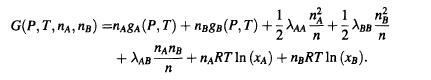
Initially, the solution has n moles of A and ng moles of B.
(a) If an amount, Ang, of B is added keeping the pressure and temperature fixed, what is the change in the chemical potential of A?
(b) For the case AAA = ABB AAB, does the chemical potential of A increase or decrease?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:






