Arrange the following compounds in order of increasing acidity. Estimate pK a values for each. (a) (b)
Question:
Arrange the following compounds in order of increasing acidity. Estimate pKa values for each.
(a)
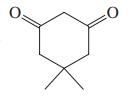
(b) CH3CO2H
(c) CH3OH
(d)
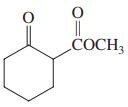
(e) CH3CHO
(f)
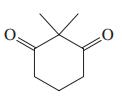
(g) CH3O2CCH2CO2CH3
(h) CH3O2CCO2CH3
Transcribed Image Text:
СОСН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)
a b CH3CO2H c CH3OH d ...View the full answer

Answered By

Sulochana kumawat
4 year experience . i am teacher . i am toutuber teaching on there / , chemistry is only my interested subject i choose this for
Teacher-Centered Methods of Instruction. Direct Instruction (Low Tech) Flipped Classrooms (High Tech) Kinesthetic Learning (Low Tech) Differentiated Instruction (Low Tech) Inquiry-based Learning (High Tech) Expeditionary Learning (High Tech) Personalized Learning (High Tech) Game-based Learning
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Arrange the following compounds in order of increasing boiling point. (b) (a) (d) (c)
-
Arrange the following compounds in order of increasing melting point. CaO HC-C-O-C-C-H i441 H-C-C-C-C-O- KCI
-
Rank the following compounds in order of increasingacidity: (a) CH3CH2CO2H (b) CH3CH2OH (c) (CH3CH2)2NH (d) CH3COCH3 (e) (f) CCI3CO2H CCH-CCH3
-
Copper Products Limited leases property on which copper has been discovered. The lease provides for an immediate payment of $472,000 to the lessor before drilling has begun and an annual rental of...
-
Which of the following can lead to a reversal of the country's trade pattern (that is, a shift in which a previously exported good becomes an imported good, or a previously imported good becomes an...
-
In the spectrum below, identify as many peaks as you can in the X-ray fluorescence spectrum of soil from the location where water from the roof of a house drips onto the ground. For each KK peak,...
-
To what extent do salaries for Strategic partner and other HR roles differ, and to what extent do they vary within Strategic partner type roles?? lop4
-
1. How might Visa executives use scenario planning in the budgeting process? 2. Could Visa have accomplished its funding goals through short-term or long-term debt financing instead? Why or why not?...
-
3) c) Dion borrows $45,000 today at the interest rate of 4% p.a. effective. He promises to make 5 yearly repayments of $10,000 starting in one year. To pay off the loan, he has decided to make an...
-
Table 1 shows Apple's online orders for the last week. When shoppers place an online order, several "recommended products" (upsells) are shown as at checkout an attempt to upsell See table 2 in cell...
-
What are a few of the typical investing activities for a company like Caesars Entertainment, developer and operator of high end hotels and casinos?
-
Give the expected results of the reaction of each of the following molecules (or combinations of molecules) with excess NaOCH 2 CH 3 in CH 3 CH 2 OH, followed by aqueous acidic work-up. (a) (b) (c)...
-
On Ma\ 10. Romano Corporation issues 1,000 shares of $10 par value common stock for cash at $18 per share. Journalize the issuance ol the stock.
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
The following program contains 9 mistakes! What are they? public class Oops2 { 2. public static void main (String [] args) { 3 int x; 4 System.out.println ("x is" x); int x = 15.2; // set x to 15.2...
-
The comparative statements of financial position of Menachem NV at the beginning and end of the year 2019 appear below. Net income of ¬34,000 was reported, and dividends of ¬23,000 were paid...
-
Draw a line-bond structure for 1, 3-butadiene, H2C = CH CH = CH2; indicate the hydribization of each carbon; and predict the value of each bond angle.
-
Following is a molecule model of aspirin (acetylsalicylic acid). Identify the hydribization of each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (gray = c. red = O, ivory...
-
Draw a line-bond structure for the propyne, CH3C CH; indicate the hydribization of each carbon; and predict a value for each bond angle.
-
solve this plz Alba Company is considering the introduction of a new product. To determine the selle price of the product you have The direct material permit The direct labor per unit The variable...
-
Calculate the current ratio collection period for accounts receivable, inventory turnover, gross margin percentage, and return on equity for 2014 and 2015 for the Jordan Corporation. Do not average....
-
A company received $11,000 cash in exchange for 200 shares of the companys common stock. What would the effect of this transaction on the current years accounting equation? Select one: A. No effect...

Study smarter with the SolutionInn App


