Both methylbenzene (toluene) and 1,6-heptadiyne have molecular formulas of C 7 H 8 and molecular masses of
Question:
Both methylbenzene (toluene) and 1,6-heptadiyne have molecular formulas of C7H8 and molecular masses of 92. Which of the two mass spectra shown below corresponds to which compound? Explain your reasoning.
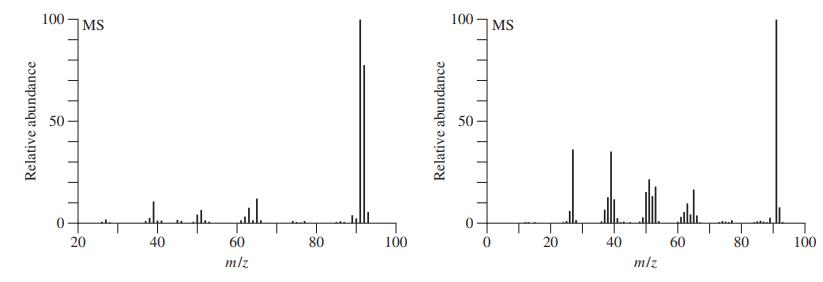
Transcribed Image Text:
100 MS 100 |MS 50 - 50 – 20 40 60 80 100 20 40 60 80 100 m/z mlz Relative abundance Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The first spectrum is for Toluene The structure elucidation is as follows mz value Fragmen...View the full answer

Answered By

KAVITA KESWANI
I have a degree of master's in chemistry which was achieved 9 years ago but at knowledge level I was not convinced with my greed of science exploration. So I went for different exam CSIR NET , GATE and many more to make myself eligible to be called as postgraduate of science. I am proud to say that I have successfully cleared both of the exams and still on the journey to achieve more.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Two spectra are shown. Propose a structure that corresponds to each spectrum. lz 50Hz (a) C3H,Cl Z50Hz 1.1 1.0 3.6 3.5 1.9 1.8 10 7 4 0 (ppm) OHz 50Hz Hz (b) CgHi002 8.0 7.98 738 726 10 7 4 0 (ppm)
-
The three compounds hexane. 2-methylpentane and 3-methylpentane correspond to the three mass spectra shown below. Match each compound with the spectrum that best fits its structure on the basis of...
-
Given the mass spectrum in Figure 9.44 and the fact that the 1H NMR spectrum for this compound consists of only a large doublet and a small septet, what is the structure of the compound? Explain your...
-
Sandys Socks makes the worlds best socks. Information for the last eight months follows: Prepare a scatter graph by plotting Sandys data on a graph. Then draw a line that you believe best fits the...
-
Describe the systems approach and its significance for project managers.
-
How do you define an array without providing a size declarator?
-
To what extent does practice match policy?? lo1
-
The auditors of SSC Company are working on both audit objectives for the various accounts and documentation requirements. Parts (a) through (d) of this question relate to objectives, while part (e)...
-
Suzanne borrowed a certain amount of money at an annual simple interest rate of 4.5%. If she returned $5338 after 184 weeks, how much interest did she pay? Round your answer to the nearest dollar.
-
BMWs Sales Reporting Practices The SEC bought an action against BMW NA for inaccurate disclosures of its retail vehicle sales volume in the United States. In order to close the gap between actual...
-
Following are spectroscopic and other data for several compounds. Propose a structure for each of them. (a) Molecular formula = C 6 H 4 Br 2 . 1 H NMR spectrum A. 13 C NMR: 3 peaks. IR: v = 745 (s,...
-
(a) Is it possible to distinguish the three isomers of dimethoxybenzene solely on the basis of the number of peaks in their proton-decoupled 13 C NMR spectra? Explain. (b) How many different isomers...
-
Suppose that the government budget is balanced (G = T), and household saving is $1 trillion. a. If this is a closed economy, what is the value of planned investment (I p )? b. If this is an open...
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
A liquid mixture of 65 mole% n-nonane and 35 mol% n-octane enters a flash unit. In the flash unit, the pressure is reduced to 1 atm and half of the liquid is evaporated. find the temperature in the...
-
To gain a deep understanding of SAPPI LIMITED's industry and competitive environment, answer the following questions before the company can embark on a "new strategy" breakaway. Does this industry...
-
What communication tools can a manager use to construct and deliver constructive and timely feedback to their employees? Discuss the various communication tools (i.e. email, phone, text, social...
-
Spalding Pointers Corporation expects to begin operations on January 1, Year 1; it will operate as a specialty sales company that sells laser pointers over the Internet. Spalding expects sales in...
-
Imagine you are the HR manager at a company, and a female employee came to you upset because she felt a male coworker was creating a hostile work environment by repeatedly asking her out on dates...
-
In his classic book Polar molecules, Debye reports some early measurements of the polarizability of ammonia. From the selection below, determine the dipole moment and the polarizability volume of the...
-
F. Luo, G.C. MeBane, 0. Kim, C.F. Giese, and W.R. Gentry (J. Chem Phys. 98,3564 (1993) reported experimental observation of the He2 complex, a species that had escaped detection for a long time. The...
-
From data in Table 18.1 calculate the molar polarization, relative permittivity, and refractive index of methanol at 20e. Its density at that temperature is 0.7914 g cm-1,
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
Kenneth lived in his home for the entire year except for when he rented his home (near a very nice ski resort) to a married couple for 14 days in December. The couple paid Kenneth $14,000 in rent for...

Study smarter with the SolutionInn App


