Compound A, C 8 H 8 O, exhibits 1 H NMR spectrum A. Upon treatment with concentrated
Question:
Compound A, C8H8O, exhibits 1H NMR spectrum A. Upon treatment with concentrated aqueous HCl, it is converted almost instantaneously into a compound that exhibits spectrum B. What is compound A, and what is the product of its treatment with aqueous acid?
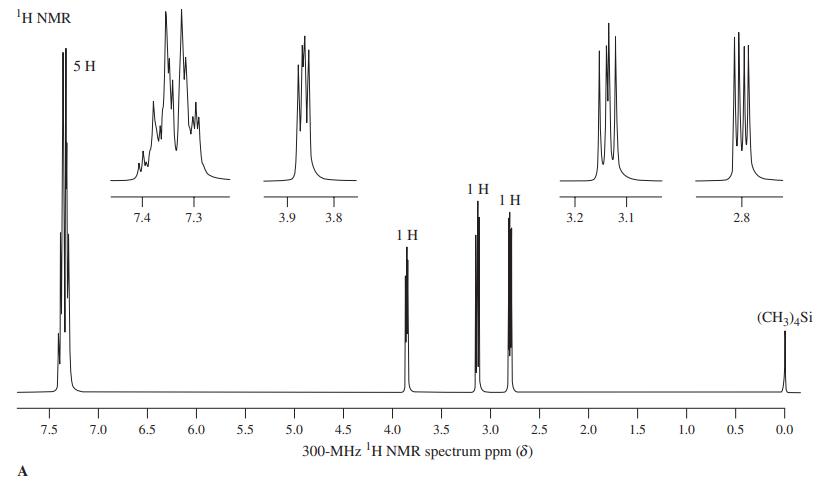
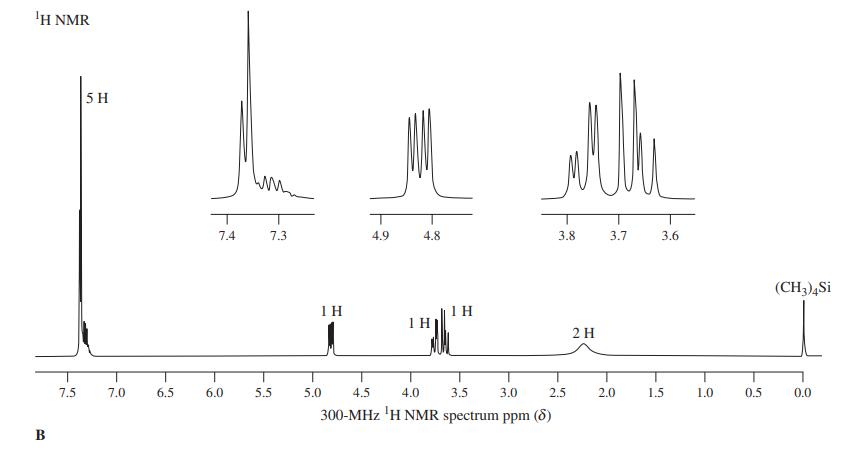
Transcribed Image Text:
'H NMR 5 H 1 H 1 H 7.4 7.3 3.9 3.8 3.2 3.1 2.8 1 H (CH3),Si 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 300-MHz 'H NMR spectrum ppm (8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
Compound A Molecnlar formula C 8 H 8 0 Degree of unsaturation 81 9 4 ...View the full answer

Answered By

Avijit Kundu
I am a Chemistry postgraduate from IIT BHUBANESWAR with CGPA 8.9.
I have experience in teaching 10th and 12th standard students as well as IIT-JEE,MEDICAL students for 2 years.Currently I am working as a Chemistry teacher in St. Xavier's high school.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Treatment of the alcohol corresponding to NMR spectrum D in Problem 47 with hot concentrated HBr yields a substance with the formula C5H11Br. Its 1H NMR spectrum exhibits signals at ( = 1.0 (t, 3 H),...
-
The 1H NMR spectrum of compound A (C8H8O) consists of two singlets of equal area at 5.1 (sharp) and 7.2 ppm (broad). On treatment with excess hydrogen bromide, compound A is converted to a single...
-
The 1H NMR spectrum of compound A (C8H8O) consists of two singlets of equal area at 5.1 (sharp) and 7.2 ppm (broad). On treatment with excess hydrogen bromide, compound A is converted to a single...
-
Mr. CFE is the sole proprietor of a hardware store called, The CFE Shop. Mr. CFE has decided to incorporate the business but wishes to minimize any income inclusions on the transfer of his business...
-
Use the data in WAGE2.RAW for this exercise. (i) In Example 15.2, using sibs as an instrument for educ, the IV estimate of the return to education is . 122. To convince yourself that using sibs as an...
-
Play Things is developing a new Lady Gaga doll. The company has made the following assumptions: The doll will sell for a random number of years from 1 to 10. Each of these 10 possibilities is...
-
If you ask users why they did not participate in requirements specification, some of the common responses are the following: a. I wasn't asked. b. I didnt have time. c. They were talking about a...
-
Plant acquisitions for selected companies are presented below. 1. Protex Inc. acquired land, buildings, and equipment from a bankrupt company, for a lump-sum price of $700,000. At the time of...
-
Hartley Electronics, Inc in Nashville, produces short runs of custom airwave scanners for the defense industry You have been asked by the owner, Janet Hartley to reduce inventory by introducing a...
-
After six months of study, much political arm wrestling, and some serious financial analysis, Dr. Martin Starr, president of Southwestern University, had reached a decision. To the delight of its...
-
At which position(s) do you expect 3-acetylquinoline (margin) to undergo nitration in the presence of a mixture of sulfuric and fuming nitric acids? Will this reaction be faster or slower than...
-
Heterocycle C, C 5 H 6 O, exhibits 1 H NMR spectrum C and is converted by H 2 and Raney nickel into compound D, C 5 H 10 O, with spectrum D. Identify compounds C and D. (Note: The coupling constants...
-
Refer to QS 4-1 and prepare journal entries to record each of the merchandising transactions assuming that the periodic inventory system is used. AppendixLO1
-
Following the example shown in (a) below, indicate the accounting effects of the listed transactions on the assets, liabilities, and stockholders' equity of Martin \& Company, a corporation: a....
-
For each transaction in E2-6A, indicate the related source document or documents that provide evidence supporting the transaction. Exercise E2-6A Unique Designs, a firm providing art services for...
-
Unique Designs, a firm providing art services for advertisers, began business on June 1. The following accounts in its general ledger are needed to record the transactions for June: Cash; Accounts...
-
The figure shows two parts of the graph of a continuous differentiable function f on [10, 4]. The derivative f is also continuous. To print an enlarged copy of the graph, go to MathGraphs.com. (a)...
-
Does your list of 10 products include items with components that are both domestically made and foreign made? Demography is the study of the structure of human populationstheir size, age composition,...
-
Write a program in a class CharacterFrequency that counts the number of times a digit appears in a telephone number. Your program should create an array of size 10 that will hold the count for each...
-
Using a graphing utility, graph y = cot -1 x.
-
As a rule, axial alcohols oxidize somewhat faster than equatorial alcohols. Which would you expect to oxidize faster, cis-4-tert-butylcyclohexanol or trans-4-tert-butylcyclohexanol? Draw the more...
-
Propose a synthesis of bicyclohexylidene, starting from Cyclohexanone as the only source of carbon. Bicyclohexylidene
-
A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate:...
-
Oscar Corporation generated $1 million of taxable income. Oscars activities and sales are restricted to State P, which imposes a 10% income tax. 100% of the stock of Oscar is owned by Felix...
-
Attempts: Keep the Highest: 12 6. Checking account reconciliation How do you balance your checkbook? Your roommate, Felix, has never before had a checking account, but he finally opened one last...
-
Spanish Peaks Railroad Inc. is considering acquiring equipment at a cost of $124,000. The equipment has an estimated life of 10 years and no residual value. It is expected to provide yearly net cash...

Study smarter with the SolutionInn App


