Formulate a mechanism for the acid-catalyzed hydrolysis of the pyrrolidine enamine of cyclohexanone (shown in the margin).
Question:
Formulate a mechanism for the acid-catalyzed hydrolysis of the pyrrolidine enamine of cyclohexanone (shown in the margin).
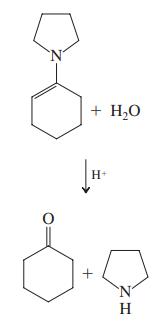
Transcribed Image Text:
+ H,O H+ 'N' H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
HOH H ...View the full answer

Answered By

Ketankumar amlani
I completed my bachelor degree in 2012 with 72.57%
i completed my master degree in 2014 with 67.50%
i completed my bachelor of education in 2019 with 87.50%
I qualified GATE (graduate aptitude test in engineering) examination in 2020
I qualified GSET (Gujarat state eligibility test) examination with highest marks in Gujarat.
I am doing personal coaching from 2014 to till date.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Formulate a mechanism for the acid-catalyzed hydrolysis of 3-methylpentanamide shown on p. 948. Data From page 948. Base Hydrolysis of an Amide II.. CH3CH2CH2CNHCH3 II.. > CH3CH2CH2CO:- + CH3NH2 II.....
-
Propose a mechanism for the acid-catalyzed hydrolysis of phenylalanine ethyl ester.
-
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with pyrrolidine.
-
McIntyre Industries Work in Process Inventory account had a $68,000 beginning balance on May 1 ($40,000 of this related to direct materials used during April, while $28,000 related to conversion...
-
A mother requested an order modifying child support after the father won an annuity worth $2 million in the state lottery. At the same time she requested the father be ordered to "pay his share of...
-
Solve by Cramers rule. Check by Gauss elimination and back substitution. Show details. 3y - 4z = 16 2x - 5y + 7z = -27
-
1 What other forms of authority has he acquired?
-
Steinberg Corporation and Dietrich Corporation are identical firms except that Dietrich is more levered. Both companies will remain in business for one more year. The companies economists agree that...
-
Problem 1 (12 points) GT Co.s accountant observes the following related to GT Co.s Cash account balance as of 10/31/21. Use this information to prepare a bank reconciliation for GT Co. GT Co.s...
-
What financial statement misrepresentations may result from an inconsistently applied credit policy? Be specific.
-
Would the use of an enamine instead of an enolate improve the likelihood of successful alkylation of a ketone by a secondary haloalkane?
-
Write the structures of the aldol condensation products of (a) pentanal; (b) 3-methylbutanal; (c) cyclopentanone.
-
Consider the reaction: If a solution initially contains 0.210 M HC 2 H 3 O 2 , what is the equilibrium concentration of H 3 O + at 25 C? HCH3O (aq) + HO(1) H3O+(aq) + CH3O (aq) K 1.8 x 10-5 at 25 C =
-
There are various compounds and epoxies that have been used to "final bed" rifle stocks. In your opinion, which is the best and why? Does this depend on the material of the stock? If so, why? Conduct...
-
Taylor Series: Problem 3 Previous Problem Problem List Next Problem 8 (5 points) Write the Taylor series for f(x) = x about x = 2 as C, (x 2)". Find the first five coefficients. n=0 - Co= C1= C2= C3=...
-
Using the figure below, draw the FBD , ?Shear Force and Bending Moment diagrams and find the maximum internal moment for the beam shown. 10 kNm 10 kN 3 m
-
Suppose the goods market is: Y = 1800 - 100i and the LM curve Y = 500 +591, where x is the last digit of your ID number. Determine the equilibrium income (Y), interest rate (i). Explain the role of...
-
Complete the chart: [1] Length, L (m) Period, T (s) LogL LogT 0.10 0.63 0.20 0.90 0.30 1.00 0.40 1.27 0.50 1.42 0.60 1.55 0.70 1.68 0.80 1.80 0.90 1.90 1.00 2.02 Plot the data T vs L. [4 (title, axes...
-
Northwest Sales had the following transactions in Year 1: 1. Acquired $200,000 cash from the issue of common stock. 2. Purchased $900,000 of merchandise for cash in Year 1. 3. Sold merchandise that...
-
The landing gear of an aircraft with: mass of 2000 kg the spring-mass-damper system Consider that the runway surface is y(t) = 0.2 cos 157.08t stiffness of the spring is 5 x 105 N/m. What is the...
-
Describe the essential features of the harpoon mechanism.
-
Describe the formulation of the Eyring equation.
-
Describe how the following techniques are used in the study of chemical dynamics: infrared chemiluminescences laser-induced fluorescence, multi-photon ionization, resonant multi-photon ionization,...
-
1. (12 pts) McNally Motors just paid the dividend of $1. The stock's dividend is expected to grow at a constant rate of 5 percent a year in to the future. The stock's discount rate is 13% A....
-
"For many companies, the recognition approach adopted by AASB16: Leases has changed their Balance Sheet." Explain to what extent you agree with this statement. Include a supporting example in your...
-
Mr. AB, a senior partner in a designing firm has a 30% share in earnings. In 2019, he transferred to the firm property with a current fair value of 25000 that originally cost him 30,000 and made a...

Study smarter with the SolutionInn App


