Rank each of the following groups of organic compounds in order of decreasing acidity. (a) (b) (c)
Question:
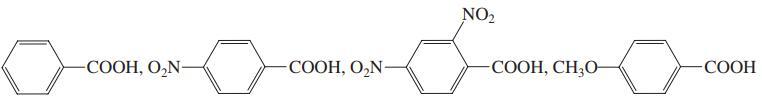
Rank each of the following groups of organic compounds in order of decreasing acidity.
(a)
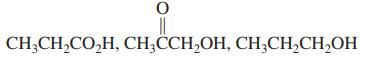
(b)
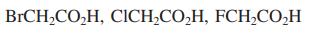
(c)
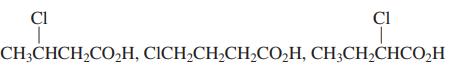
(d)
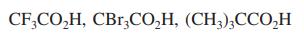
(e)
Transcribed Image Text:
CH;CH,CO,H, CH;CCH,OH, CH;CH,CH,OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
a Pka of Propanol 16 Therefore the order decreasing acidity CH 3 CH 2 COOH CH 3 CCH 2 OH CH 3 CH 2 CH 2 OH b BrCH 2 COOH Cl CH 2 COOH FCH 2 COOH We kn...View the full answer

Answered By

SUMANT JHA
I am a Metallurgical Engineering graduate with an aggregate of 83.10%. I did my final year project from the reputed research centre of India ie, BARC on Austenitic Stainless steels.I completed my Senior Secondary Exam with an aggregate of 83.40%.
I started teaching from 2013 giving home tution to the students in Mathematics , Physics and Chemistry so till now i have seven years of experience of teaching these subjects.I also started teaching subjects of Material Science Engineering from 2018. I have assisted many students in preparing for Engineering Entrances Examination. My prime focus in tutoring is to clear the basic concepts of the subjects to the students because while i was a student i felt that clear concept is required for the deep study of the subjects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Rank the following compounds in order of decreasing acidity of the indicated hydrogen: O 0 CH CCH2CH2CCH3 CH CCH2CH2CH2CCH3 CH CCH2CCH3
-
Rank the compounds in order of decreasing λ max: CH CH CH CH2
-
Rank each of the following sets of molecules in order of increasing SN2 reactivity. (a) CH3CH2Br, CH3Br, (CH3)2CHBr (b) (CH3)2CHCH2CH2Cl, (CH3)2CHCH2Cl, (CH3)2CHCl (c) (d) CH,CH,CI, CH,CH2I. CI...
-
A recent PwC Supply Global Chain survey indicated that companies that acknowledge the supply chain as a strategic asset achieve 70% higher performance. The Leaders in the survey point to...
-
In February 1996, President Bill Clinton signed the Telecom munications Act, which (among many other things) required all TV sets sold in the United States to be equipped with a V-chip, which allows...
-
Which step of the systems development life cycle (SDLC) reviews issues with a current system and establishes the requirements of the new system being created? a. Maintenance and change b....
-
(Debtor/Creditor Entries for Settlement of Troubled Debt) Petra Langrova Co. owes $199,800 to Mary Joe Fernandez Inc. The debt is a 10-year, 11% note. Because Petra Langrova Co. is in financial...
-
1. What type of data source would the companys own historical data be? Identify other possible data sources that the research team might use to examine the current marketplace for residential...
-
Modem Appliances, Inc. sells food processors for $150 with a 120-day warranty against defects. Past experience indicates that 5% of the processors will have some defect during the warranty period and...
-
Grinzaid Home Nursing specializes in home nursing services. It bases its overhead on the flexible budget cost function of $33,000 + ($2.40 labor hours). Normal volume is based on 12,000 labor hours....
-
What is the IUPAC name of the compound shown? (a) (E )-3-Methyl-2-hexenoic acid (b) (Z )-3-Methyl-2-hexenoic acid (c) (E )-3-Methyl-3-hexenoic acid (d) (Z )-3-Methyl-3-hexenoic acid H;C CO.H C=C...
-
Select the acid with the highest K a (i.e., lowest pKa). (a) H 3 CCO 2 H (b) (c) (d) (d) Cl 2 CHCO 2 H CO,H I
-
The following data were obtained from the 2016 financial statements for two U.S. automakers (in millions of dollars): Required 1. Calculate and interpret total productivity in dollars for the two...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
Below are incomplete financial statements for Hurricane, Incorporated Required: Calculate the missing amounts. Complete this question by entering your answers in the tabs below. Income Statement Stmt...
-
TBTF Incorporated purchased equipment on May 1, 2021. The company depreciates its equipment using the double-declining balance method. Other information pertaining to the equipment purchased by TBTF...
-
Coco Ltd. manufactures milk and dark chocolate blocks. Below is the information relating to each type of chocolate. Milk Chocolate Selling price per unit $6 Variable cost per unit $3 Sales mix 4 Dark...
-
Data related to 2018 operations for Constaga Products, a manufacturer of sewing machines: Sales volume 5,000 units Sales price $300.00 per unit Variable production costs Direct materials 75.00 per...
-
Write a web page with embedded PHP to print the first 10 rows of Pascals triangle (see Example C 17.10 if you dont know what this is). When rendered, your output should look like Figure 14.21. Figure...
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
Bromine is larger than chlorine, yet the two atoms have identical axial destabilization energies. Explain.
-
Draw the stereo isomers of these compounds: (a) 1, 3-Dimethyleyclohexane (b) 1, 2-Diethylcycloproane (c) 1-Chloro-3-methylcyclopentane
-
Draw both chair conformations of trans-1, 3-dimethyl cyclohexane indicate whether each methyl group is axial or equatorial.
-
Suppose you have the following information: 1 USD = 0.689 GBP 1 USD = 1.346 CAD What is the GBP/CAD cross rate? Enter your answer rounded off to FOUR decimal points. Do not enter any currency sign in...
-
Concord Corporation constructed a building at a cost of $21,000,000. Weighted-average accumulated expenditures were $13,200,000, actual interest was $1,240,000, and avoidable interest was $71,000. If...
-
If the expected rate of return on a portfolio consisting of two securities is 18.8% and the return on one security, which constitutes 30% of the portfolio is 16%, the return on the other security is...

Study smarter with the SolutionInn App


