Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic aromatic
Question:
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic aromatic substitution. Explain your answers.
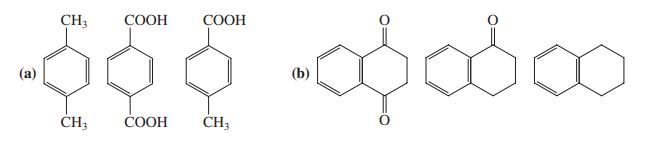
Transcribed Image Text:
CH3 СООН СООН (а) (b) ČH; СООН ČH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
A Benzene undergoes electrophilic substitution reaction and presence of electron donating groupsEDG ...View the full answer

Answered By

VINOD BANSAL
I have completed my Post Graduation in Chemistry from Indian Institute Of Technology BOMBAY (IITB) and my graduation B.Sc(H) in Chemistry from HINDU COLLEGE, UNIVERSITY OF DELHI,DELHI. I am working as Chemistry faculty for IIT JEE MAINS, ADVANCE AND NEET with one of the best coaching institute i.e PACE IIT & MEDICAL.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
List the compounds in each of the following groups in order of decreasing acidity: a. b. c. CH2-CH2 CH3CH3 CH3CH HC=CH 0 O CH CCH,CCH CH CCH2COCH
-
Rank the compounds in each group in order of decreasing acidity. (a) CH3CHCICH20H, CH3CHBrCH2OH, BrCH2CH2CH2OH (b) CH3CH2CH2OH, CCI3CH2OH, (CH3)2CC1CH2OH (c) (CH3)2CHOH, (CF3)2CHOH, (CC13)2CHOH
-
Rank the compounds in order of decreasing λ max: CH CH CH CH2
-
Prepare the journal entries for 2, 4, 6, 8, 12 and 14 using the following information 6% bonds $1200000 par value payable in 5 years were issued for cash at 108, any premium is to be transferred to...
-
Read the case file at the beginning of this chapter. Compare and contrast the case file facts with the facts of the Mapp case. Look for similar facts, differences and factual gaps.
-
Look at the following code, which is the first line of a class definition: Public class Tiger extends Felis In what order will the class constructors execute?
-
1 Gather other evidence of changes in working practice, and decide whether it supports or contradicts Herzbergs theory.
-
Penny Francis inherited a $100,000 portfolio of investments from her grandparents when she turned 21 years of age. The portfolio is comprised of the following three investments: a. Based on the...
-
With a straight deductible , the insured must pay a certain number of dollars of loss before the insurer is required to make a payment e.g., an auto insurance deductible You insured your car with 500...
-
A Cassegrain astronomical telescope uses two mirrors to form the image. The larger (concave) objective mirror has a focal length Æ1 = +50.0 cm. A small convex secondary mirror is mounted 43.0...
-
Specify whether you expect the benzene rings in the following compounds to be activated or deactivated. NO2 OH (a) (b) 'NO2 CH3 COOH NH2 SO-H HO C(CH3)3 (d) (e) (f) 'NO2 H3
-
Halogenation of 1,3-dimethylbenzene (m-xylene) takes place 100 times faster than halogenation of either its 1,2- or 1,4-isomers (o- or p-xylene). Suggest an explanation.
-
Preliminary plans are underway for construction of a new stadium for a major league baseball team. City officials question the number and profitability of the luxury corporate boxes planned for the...
-
James Cook, a production department worker, is paid on hourly basis at a rate of $15 per hour. James works 40 hours per week. Any time James works over 40 hours, it is considered as overtime and he...
-
You just started working as a Health Service Manager within one of the following healthcare industries. First, choose an industry below to discuss the questions that follow: Ambulatory Surgery center...
-
Q10: Region ( experienced compressive stresses and has a than the rest of the bracket. Region ( ) experienced tension stresses and has a of the bracket. Deep Drawing and Stretch Forming width (into...
-
A sample of 1500 computer chips revealed that 32% of the chips do not fail in the first 1000 hours of their use. The company\'s promotional literature claimed that above 29% do not fail in the first...
-
The 75 lb block is released from rest 5 ft above the plate. Determine the compression of each spring when the block momentarily comes to rest after striking the plate. Neglect the mass of the plate....
-
The following information relates to Home Depot, Inc., and Lowes Companies, Inc. for their 2017 and 2016 fiscal years. Required a. Compute the following ratios for the companies 2017 fiscal years...
-
Suppose the S&P 500 futures price is 1000, = 30%, r = 5%, = 5%, T = 1, and n = 3. a. What are the prices of European calls and puts for K = $1000? Why do you find the prices to be equal? b. What...
-
At an altitude of 15 km the temperature is 217 K and the pressure 12.1 kPa. What is the mean free path of N, molecules? (a= 0.43 NM2)
-
How many collisions per second does an N2 molecule make at an altitude of 15 km? (See Exercise 21.4b for data.)
-
Calculate the mean free path of carbon dioxide molecules using a= 0.52 nm at 25C and (a) 15 atm, (b) 1.0 bar, (c) 1.0 Torr.
-
Consider the following income statement. Please use excel. You have to use the Percentages. Income Statement - Seagate Technology ( in millions ) 7 / 1 / 2 0 2 2 Revenue $ 1 1 , 6 6 1 . 0 Cost of...
-
The real risk-free rate is 3.4%, and inflation is expected to be 3.8% for the next 2 years. A 2-year Treasury security yields 7.3%. What is the maturity risk premium for the 2-year security? Round to...
-
How is fraud risk influenced by a companys internal controls? How is fraud risk influenced by a companys ethics, values, and expectations?

Study smarter with the SolutionInn App


