Specify whether you expect the benzene rings in the following compounds to be activated or deactivated. NO2
Question:
Specify whether you expect the benzene rings in the following compounds to be activated or deactivated.
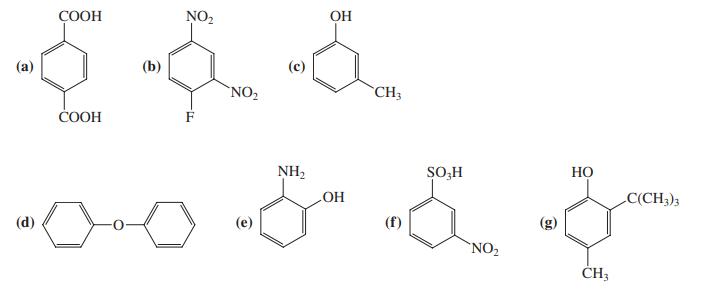
Transcribed Image Text:
СООН NO2 OH (a) (b) 'NO2 CH3 COOH NH2 SO-H Но HO C(CH3)3 (d) (e) (f) 'NO2 ČH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a In compound a COOH group is deactivating Hence benzene ring is expect...View the full answer

Answered By

Aditi Deswal
Currently , I am doing post graduation ( MSc.) in Chemistry . I want to bacome a lecturer . I feel happy when I teach student and solve their problem . It is my passion as well as hobby . I feel blessed If I got a chance to share my knowledge as much as I have . I teach student at my home also. I love teching and want to spend my whole life to teach the students and explores new things.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position...
-
Biochemical oxidation of aromatic rings is catalyzed by a group of liver enzymes called aryl hydroxylases. Part of this chemical process is the conversion of toxic aromatic hydrocarbons such as...
-
The following compound has a pentasubstituted benzene ring. (a) Starting with benzene and using any other necessary reagents of your choice, design a synthesis for this compound. (b) It is very...
-
What is meant by date alignment? Does it exist on the consolidated worksheet under the following methods, and if not, how is it created prior to elimination of the investment account under each of...
-
During a criminal trial for extortion, a psychiatrist testified regarding defendant. His testimony and opinion were based primarily on information and reports from other doctors. Defendant was...
-
Here is the first line of a class declaration. What is the name of the superclass? What is the name of the subclass? Public class Truck extends Vehicle
-
1 You get where you are in business because of other people. Why not put the business back in the hands of the people who helped build it? Mike Thompson established Child Base Nurseries in 1989, and...
-
Cost Determination, LCM, Retail Method Olson Corporation, a retailer and wholesaler of national brand-name household lighting fixtures, purchases its inventories from various suppliers. (a) (1) What...
-
Alicia has been working for JMM Corp. for 33 years. Alicia participates in JMMs defined benefit plan. Under the plan, for every year of service for JMM she is to receive 2 percent of the average...
-
There must be something wrong with these statements! exclaimed Hugh Richards, president of Ajax Inc. They just dont make sense. We sold the same number of units this year as we did last year, yet our...
-
The rate of nitration of (chloromethyl)benzene, shown below is 0.71 relative to the rate of nitration of benzene (=1). The (chloromethyl)nitrobenzene product mixture that results contains 32% ortho,...
-
Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic aromatic substitution. Explain your answers. CH3 () (b) H; H3
-
How does modern relativity modify the law of conservation of momentum?
-
A vertical solid cylinder of uniform cross-sectional area A floats in water. The cylinder is partially submerged. When the cylinder floats at rest, a mark is aligned with the water surface. The...
-
Non-manufacturing fixed cost for year 2011 equal to:$60,780 out of which half are Administrative expenses.Administrative expenses are expected to increase by: 10%The total Variable nonmanufacturing...
-
Q13. The probability that Ryan will roll a three using a standard die is 1/6. Let Y = number of times that Ryan has to roll a die in order to roll the first three. What is the expected value for Y?...
-
1. The following are data for two IT projects for a new database system. Prepare a spreadsheet for two projects, using the following data. Amounts are in thousands of dollars. Calculate the NPV for...
-
The Matsui Lubricants plant uses the weighted-average method to account for its work-in-process inventories. The accounting records show the following information for a particular day: Beginning WIP...
-
Presented here is selected information from the 2016 fiscal-Year 10-K reports of four companies. The four companies, in alphabetical order, are: AT&T, Inc., a company that provides communications and...
-
How does Kant answer Humes bundle theory of self? Do you think he is successful?
-
Determine the ratios of (a) The mean speeds, (b) The mean kinetic energies of His atoms and Hg atoms at 25e.
-
The best laboratory vacuum pump can generate a vacuum of about 1 n Torr. At 25C and assuming that air consists of N, molecules with a collision diameter of395 pm, calculate (a) The mean speed of the...
-
At what pressure does the mean free path of argon at 25C become comparable to the diameters of the atoms themselves?
-
For each of the following users of accounting, identify if the user would use financial accounting (FA) or managerial accounting (MA). Question content area bottom Part 1 a. Investor b. Banker c....
-
Wine and Roses Incorporated, offers a bond with a coupon of 7.5 percent with semiannual payments and a yield to maturity of 8.16 percent. The bonds mature in 10 years. What is the market price of a...
-
Kingbird Co. purchased and as a factory site for $416,000. The process of tearing down two old buildings on the site and constructing the factory required 6 months. The company paid $43,680 to taze...

Study smarter with the SolutionInn App


