Show how the pI values for the amino acids in Problem 32 (see Table 26-1) are derived.
Question:
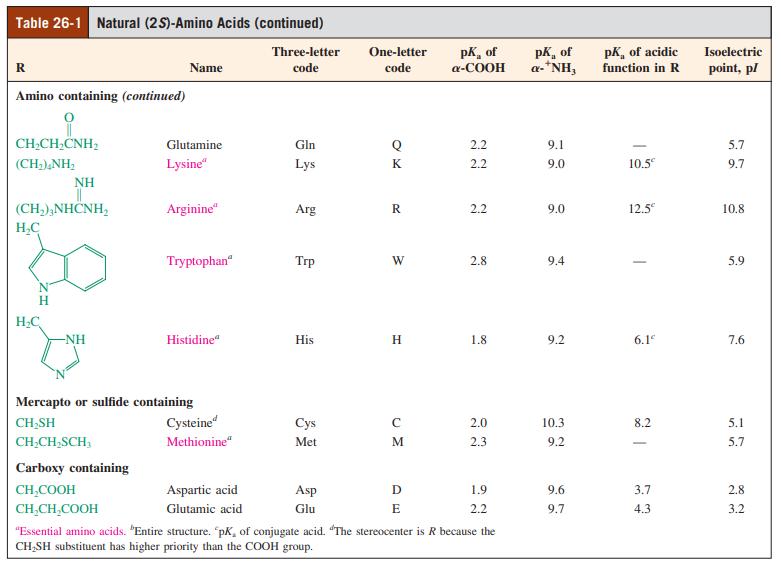
Show how the pI values for the amino acids in Problem 32 (see Table 26-1) are derived. For each amino acid that possesses more than two pKa values, give the reason for your choice when calculating pI.
Problem 32(Table 26-1)
Transcribed Image Text:
Table 26-1 Natural (25)-Amino Acids (continued) pK, of a-COOH pK, of a-*NH, pk, of acidic Three-letter One-letter Isoelectric R Name code code function in R point, pl Amino containing (continued) CH,CH,CNH, Glutamine Gln Q 2.2 9.1 5.7 (CH),NH, Lysine" Lys K 2.2 9.0 10.5 9.7 NH (CH,),NHČNH, Arginine" Arg R 2.2 9.0 12.5 10.8 H,C Tryptophan" Trp W 2.8 9.4 5.9 H. H.C -NH Histidine" His H 1.8 9.2 6.1 7.6 Mercapto or sulfide containing CH,SH Cysteine" Cys 2.0 10.3 8.2 5.1 CH;CH,SCH, Methionine“ Met M 2.3 9.2 5.7 Carboxy containing CH,COOH Aspartic acid Asp 1.9 9.6 3.7 2.8 CH,CH,COOH Glutamic acid Glu E 2.2 9.7 4.3 3.2 "Essential amino acids. "Entire structure. pK, of conjugate acid. "The stereocenter is R because the CH,SH substituent has higher priority than the COOH group.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
In the case of two pKa values the simple arithmetic mean of these two ...View the full answer

Answered By

ALOK SINGH
Hi, I completed my last degree certificate (Ph.D. in Science) in 2009. After the completion of my Master's degree (M.Sc.), I attached myself to tutoring needy students. I have more than 15 years of teaching experience. I teach students who are preparing for the Medical entrance examination. I have my own YouTube channel and have more than 40K subscribers.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Group the amino acids in Problem 32 according to whether they are (a) Positively charged, (b) Neutral, (c) Negatively charged at pH = 7. Data From Problem 32 (a) Alanine at pH = 1, 7, and 12; (b)...
-
Indicate which of the amino acids in Problem 32 and the peptides in Problem 42 would migrate in an electrophoresis apparatus at pH = 7 (a) Toward the anode (b) Toward the cathode. Data From Problem...
-
In addition to the conventional 20 amino acids in Tables 26-1 and 26-3, two others, selenocysteine (Sec) and pyrrolysine (Pyl), are incorporated into proteins using the nucleic acidbased cell...
-
The current quoted price of a 13% coupon bond is $110. It pays coupon semi-annually. The next coupon will be paid in 6-days (total number of days in this semi-annual period is 181) and the futures...
-
Add the interaction term unionit t to the equation estimated in Table 14.2 to see if wage growth depends on union status. Estimate the equation by random and fixed effects and compare the results.
-
a. If you talk to a broker selling the high-fee mutual fund, what will he or she probably tell you when you ask them, Am I getting my moneys worth when I pay your high fees? b. According to Figure...
-
Explain why in-house development projects are always time-and-materials projects. LO.1
-
Surat Limited paid cash to acquire an aircraft on January 1, 2017, at a cost of 30,000,000 rupees. The aircraft has an estimated useful life of 40 years and no salvage value . The company has...
-
Some stockholders have a preemptive right that allows them to maintain their ownership percentage in the company by purchasing additional shares of any new stock issues. Rights offerings, like...
-
9.93 Monensin is a potent antibiotic compound isolated from Streptomyces cinnamonensis. The following reaction was employed dur- ing W. C. Still's synthesis of monensin (J. Am. Chem. Soc. 1980, 102,...
-
Draw the structure that each of the following amino acids would have in aqueous solution at the indicated pH values. (a) Alanine at pH = 1, 7, and 12; (b) Serine at pH = 1, 7, and 12; (c) Lysine at...
-
Show how Hell-Volhard-Zelinsky bromination followed by amination can be used to synthesize each of the following amino acids in racemic form: (a) Gly; (b) Phe; (c) Ala.
-
Prove that in the H = LU factorization of a regular upper Hessen berg matrix, the lower triangular factor L is bidiagonal, as in (1.67). L= 4 1 12 1 1-2 -1 1 U = d d u dz uz dn' (1.67)
-
Southco is a medium-sized American-owned global manufacturer of access hardware solutions, such as latches and hinges, used for applications in the aircraft, railway, computer and automotive...
-
To what extent do staffing processes at the Dionysos reflect the strategic approach to recruitment and selection encapsulated by the conceptual framework and model depicted in Key Concepts 8.4 and...
-
Im an accounting major, not an operations expert, yelled just-promoted Bob Barthrow, the executive vice president of the Midwest Frequent Flyer Call Center, during a senior-level management meeting....
-
The Hudson Jewelers case study can be found in Appendix C. Chapter 14 Case Question for Discussion: 1.Customer demand (weekly visits) at Hudson Jewelers is highly seasonal, as shown in the worksheet...
-
Jasmine Minoza, the chief information officer of a Canada- based designer of video games, Adventure Gaming, Inc. (AGI), is considering outsourcing her companys software development activities to...
-
Create a program that tests the class Merlin described in the previous exercise. Use the toString method to verify that a unique instance has been created. Previous exercise. Sometimes we would like...
-
What steps must a business take to implement a program of social responsibility?
-
Propose structures for the following: (a) A ketone, C4H8O (b) A nitrile, C5H9N (c) A dialdehyde, C4H6O2 (d) A bromoalkene, C6H11Br (e) An alkane, C6H14 (f) A cyclic saturated hydrocarbon, C6H12 (g) A...
-
Draw as many compounds as you can that fit the following descriptions: (a) Alcohols with formula C4H10O (b) Amines with formula C5H13N (c) Ketones with formula C5H10O (d) Aldehydes with formula...
-
Draw compounds that contain the following: (a) A primary alcohol (b) A tertiary nitrile (c) A secondary Thiol (d) Both primary and secondary alcohols (e) An isopropyl (f) A quaternary carbon
-
Read the situations and answer the questions. Make reference to the UCC Article 3 in your response. Mary Smithfield gives her boyfriend Bobby a check for $100 for a birthday present. She signs the...
-
as a, ! S p. 522. B Define each term. 1. plateau 2. Mediterranean climate 3. wadi 4. steppes 5. fertile sen 6. natural resources 7. zionism 8. mosque C Identify the term described by each clue. 1. a...
-
Selected comparative statement data for Carla Vista Corporation are presented below. All balance sheet data are as at December 31. Net sales Profit for the year Total assets Total common...

Study smarter with the SolutionInn App


