Show how you would synthesize each of the following molecules from an alkene of appropriate structure (your
Question:
Show how you would synthesize each of the following molecules from an alkene of appropriate structure (your choice).
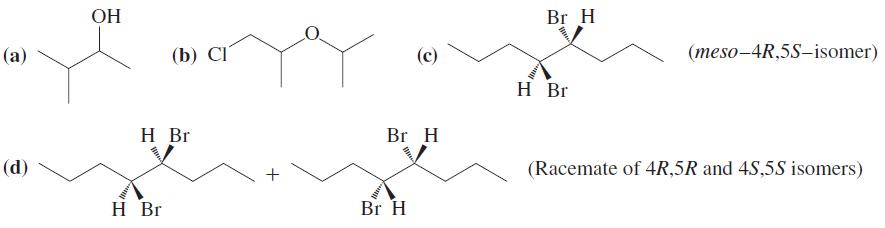
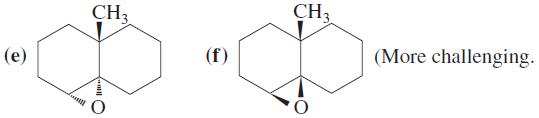
Transcribed Image Text:
ОН Br H (а) (b) СI (meso-4R,5S-isomer) H Br H Br Br H (d) (Racemate of 4R,5R and 4S,5S isomers) H Br Br H +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a b c d e f 1...View the full answer

Answered By

Nikhil Sharma
I am teaching for 4 years and as a result of that I have very clear idea about the student's difficulties and I know how to rectify them. I have deep knowledge of my subject as I have done Masters in it for finest institute of the country.
I have cleared various nation level competitive exams with very good ranks so I can help students to develop competitive attitude.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Starting with benzyl bromide, show how you would synthesize each of the following: (a) (b) (c) (d)
-
Show how you would synthesize the following alcohols from appropriate alkenes. (a) (b) (c) (d) ,
-
Show how you would synthesize each compound from starting materials containing no more than six carbon atoms. (a) (b) (c) CH2CH CHC CH
-
An analog filter is defined by its transfer function: 100n H(s) = %3D s+ 100n What type of filter H(s) is? A. Low-pass B. High-pass C. Band-pass D. Band-stop
-
Describe the process of building a probability and impact matrix and how the results are used to aid risk management planning.
-
Describe maximum parsimony.
-
Do you feel that Eric was being exploited by the personnel manager? ? LO1
-
Closing down divisions. Aristide corporation has four operating divisions. During the first quarter of 2009, the company reported total income from operations of $61,000 and the following results for...
-
Evaluate the concept of Fair Value Measurement in accounting standards. How is fair value determined, and what are the challenges associated with its application
-
You have learned the basics of investment and retirement planning. Your investing project includes everything you have learned the whole semester. Below you have a scenario. You are to evaluate your...
-
Formulate the product(s) that you would expect from each of the following reactions. Show stereochemistry clearly. Ch (b) trans-3-Heptene HCI Br2, H2O (a) (c) 1-Ethylcyclohexene CH,CH3 CH3 NaOH, H,O...
-
Propose efficient methods for accomplishing each of the following transformations. Most will require more than one step. Br () (b) (meso-2R,3Sisomer) () (Racemate of 2R,3R and 2S,3S isomers)...
-
A person who trades in his 15-year-old automobile and purchases a new car with disk brakes is practicing which method of managing risk?
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
A baseball player's slugging percentage SLG can be calculated with the following formula (which is an example of a rational function): SLG = H+2B+2x(3B)+3x(HR) AB Q Image transcription text H+2B+2x...
-
Question During 2021, Cassandra Albright, who is single, worked part-time at a doctor's office and received a W-2. She also had a cash-basis consulting practice that had the following income and...
-
Shelly Beaman (social security number 412-34-5670) Is single and resides at 540 Front Street, Ashland, NC 27898. Shelly's W-2 wages Federal withholding Social security wages Social security...
-
Modify your pow method from Exercise 5 to make a new method called pow2 that uses the type double for the first parameter and that works correctly for negative numbers. For example, the call...
-
The trade-off theory relies on the threat of financial distress. But why should a public corporation ever have to land in financial distress? According to the theory, the firm should operate at the...
-
Sketch the phase diagram for the Mg/Cu system using the following information: Br(Mg) = 648C, Br(Cu) = 1085C; two intermetallic compounds are formed with Br(MgCu2) = 800C and Br(Mg2Cu) = 580C;...
-
The temperature-composition diagram for the Ca/Si binary system is shown in Fig. 6.46. (a) Identify eutectics, congruent melting compounds, and incongruent melting compounds. (b) If a 20 per cent by...
-
Show that two phases are in thermal equilibrium only if their temperatures are the same.
-
Parker Plastic, Incorporated, manufactures plastic mats to use with rolling office chairs. Its standard cost information for last year follows: Standard Quantity Standard Price ( Rate ) Standard Unit...
-
If we add the favourable variances to the budgeted profit, and then deduct the adverse variances, which one of the following does this equal? A. Actual profit B. Flexed profit C. Zero D. Original...
-
In addition to the sales reflected in the table above, the Howells provided you with the following additional information concerning 2 0 2 3 : The Howells received a Form 1 0 9 9 - B from the...

Study smarter with the SolutionInn App


