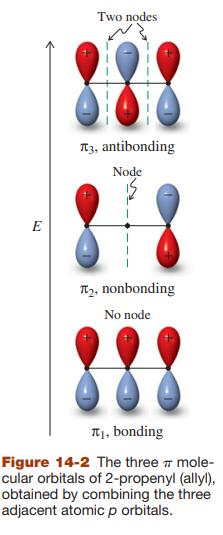
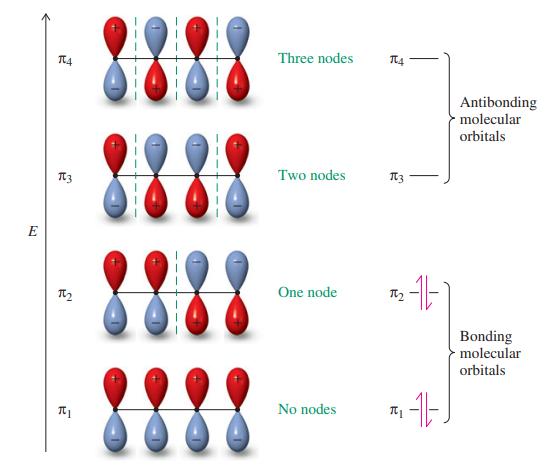
Sketch the molecular orbitals for the pentadienyl system in order of ascending energy (see Figures 14-2 and
Question:
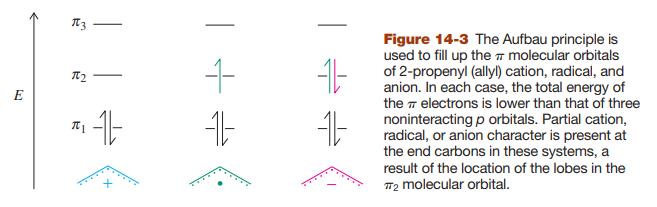
Sketch the molecular orbitals for the pentadienyl system in order of ascending energy (see Figures 14-2 and 14-7). Indicate how many electrons are present, and in which orbitals, for (a) the radical; (b) the cation; (c) the anion (see Figures 14-3 and 14-7). Draw all reasonable resonance forms for any one of these three species.
Figure 14-2, 14-3, & 14-7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:




