Arrange the following carbocations in order of decreasing stability. Draw all possible resonance forms for each of
Question:
Arrange the following carbocations in order of decreasing stability. Draw all possible resonance forms for each of them.
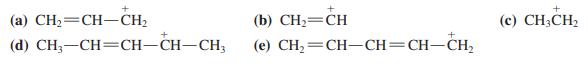
Transcribed Image Text:
(a) CH2=CH–CH2 (b) СH— СH (c) CH3CH2 (d) CH, —СH—CH-CH—CH, (e) CH,=CH-CH=CH-CH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
No of resonance str and I effect X stability Decreas...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
List the carbocations in order of decreasing stability. a. b. CH CH;CH2CHs CH3CH2CHCH CH,CH2CH2CH2 CH,CHCH,CH, 3 CH3CHCH2CH2 CH3CHCH2CH2 Ci CH
-
Rank the following carbocations in decreasing order of stability. CH3 H CH H CH
-
List the following carbocations in decreasing order of their stability. CH +CH, +CH, CH CH
-
What is the discount yield, bond equivalent yield, and effective annual return on a $ 5 million commercial paper issue that currently sells at 98.625 percent of its face value and is 136 days from...
-
Explain why the contents of a business case might change, depending on the project.
-
Find the regression line of y on x for the data (x, y) = (0, 4), (2, 0), (4, -5), (6, -9), (8, -10).
-
Set out the different elements that can make up a reward package? LOP4
-
Sequel Theatre, owned by Nadia Wood, is unique as it shows only movies that are part of a theme with sequels. As at April 30, 2017, the ledger of Sequel Theatre showed the following: Cash $18,900,...
-
Please write the journal entries for both! With the work please! 25. The Company issued $400,000 of 20 year 6.5% bonds at 97.5 . 26. Assume the same facts in #25 above except that the bonds were...
-
For the system shown in Fig. 8.17, compute the power delivered by the pump to the water to pump 50 gal/min of water at 60F to the tank. The air in the tank is at 40 psig. Consider the friction loss...
-
Give the products expected from reaction of deuterium iodide (DI) with (a) 1,3-cycloheptadiene; (b) trans-1,3-pentadiene; (c) 2-methyl-1,3-pentadiene. In what way does the observable result of...
-
Sketch the molecular orbitals for the pentadienyl system in order of ascending energy (see Figures 14-2 and 14-7). Indicate how many electrons are present, and in which orbitals, for (a) the radical;...
-
IPOs-initial public offerings of stock-create billions of dollars of new wealth for owners, managers, and employees of companies that were previously privately owned. Nevertheless, hundreds of large...
-
As machines get older, the cost of maintaining them tends to increase. Suppose for a particular machine, the rate at which the maintenance cost is increasing is approximated by the function C' (t) =...
-
At Edsel Automotive, the management team is planning to expand one of its plants by adding a new assembly line for sport utility vehicles (SUVs). The cost of setting up the new SUV assembly line is...
-
Write out the form of the partial fraction decomposition of the function (See Example). Do not determine the numerical values of the coefficients. (If the partial fraction decomposition does not...
-
Listed here are the costs associated with the production of 1,000 drum sets manufactured by TrueBeat. Costs 1. Plastic for casing$16,000 2. Wages of assembly workers$83,000 3. Property taxes on...
-
The diagram shows the instant when a long slender bar of mass 4.8 kg and length 2.9 m is horizontal. At this instant the mass m= 6.2 kg has a vertical velocity of 5.3 m/s. If the pulley has...
-
Use the class ClassObjectIODemo shown in Listing 10.10 of Chapter 10 to create a file of Species objects. The class Species is given in Chapter 10, Listing 10.9. Then write a program that reads the...
-
The May 2014 revenue and cost information for Houston Outfitters, Inc. follow: Sales Revenue (at standard).............. $ 540,000 Cost of Goods Sold (at standard) ..........341,000 Direct Materials...
-
The chemical shift of the CH3 protons in diethyl ether is, = 1.16 and that of the CH2 protons is 3.36. What is the difference in local magnetic field between the two regions of the molecule when the...
-
Sketch the appearance of the IH-NMR spectrum of diethyl ether using J = 6.97 Hz and the data in Exercise 15.9b in a spectrometer operating at (a) 350 MHz, (b) 650 MHz.
-
Two groups of protons are made equivalent by the isomerization of a fluxional molecule. At low temperatures, where the interconversion is slow, one group has 0=5.5 and the other has 0=6.8. At what...
-
The following information was available for the year ended December 31, 2022: Net sales $ 300,000 Cost of goods sold 210,000 Average accounts receivable for the year 15,000 Accounts receivable at...
-
Jeannie is an adjunct faculty at a local college, where she earned $680.00 during the most recent semimonthly pay period. Her prior year-to-date pay is $18,540. She is single and has one withholding...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...

Study smarter with the SolutionInn App


