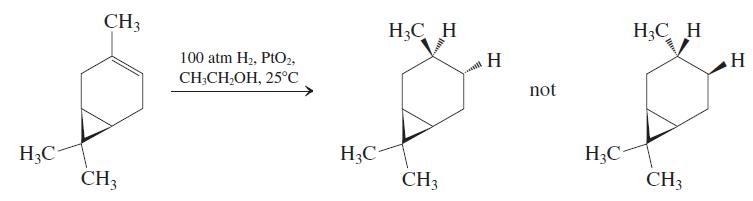
The bicyclic alkene car-3-ene, a constituent of turpentine, undergoes catalytic hydrogenation to give only one of the
Question:
The bicyclic alkene car-3-ene, a constituent of turpentine, undergoes catalytic hydrogenation to give only one of the two possible stereoisomeric products. The product has the common name cis-carane, indicating that the methyl group and the cyclopropane ring are on the same face of the cyclohexane ring. Suggest an explanation for this stereochemical outcome.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





