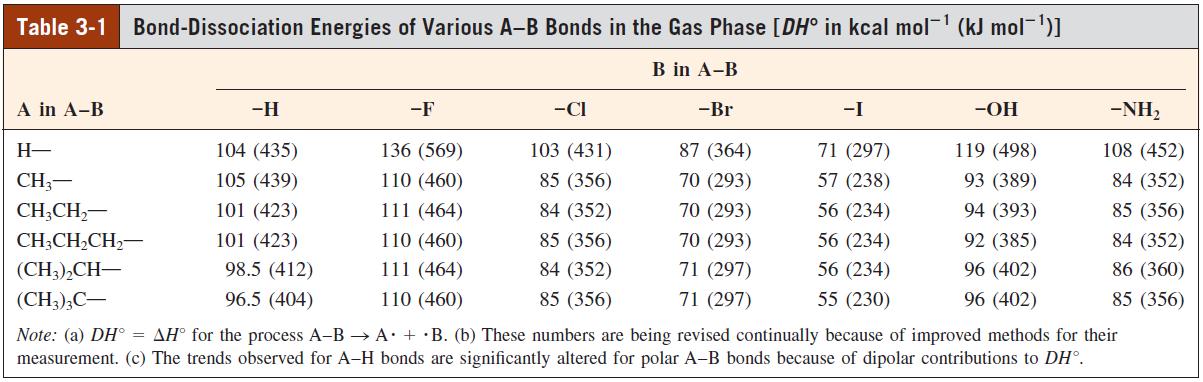
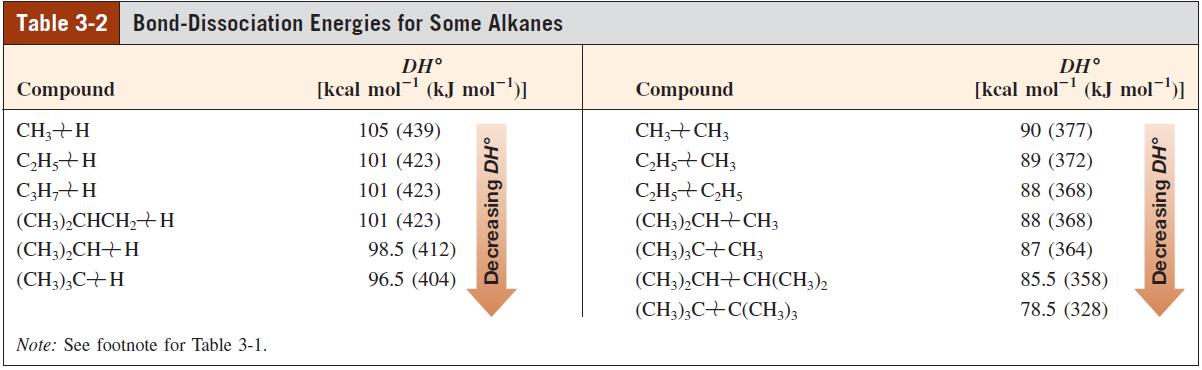
With the help of the DH values given in Tables 3-1 and 3-4, calculate the H values
Question:
With the help of the DHº values given in Tables 3-1 and 3-4, calculate the ΔHº values for addition of each of the following molecules to ethene, using 65 kcal mol-1 for the carbon – carbon π bond strength.
(a) Cl2
(b) IF (DHº = 67 kcal mol-1)
(c) IBr (DHº = 43 kcal mol-1)
(d) HF
(e) HI
(f) HO – Cl (DHº = 60 kcal mol-1)
(g) Br – CN (DHº = 83 kcal mol-1; DHº for Csp3 – CN = 124 kcal mol-1)
(h) CH3S – H (DHº = 88 kcal mol-1; DHº for Csp3 – S = 60 kcal mol-1)
Transcribed Image Text:
Table 3-1 Bond-Dissociation Energies of Various A-B Bonds in the Gas Phase [DH° in kcal mol- (kJ mol-1)1 B in A-B A in A-B -H -F -CI -Br -I -OH -NH, H- 104 (435) 136 (569) 103 (431) 87 (364) 71 (297) 119 (498) 108 (452) CH3- 105 (439) 110 (460) 85 (356) 70 (293) 57 (238) 93 (389) 84 (352) CH;CH,- 101 (423) 111 (464) 84 (352) 70 (293) 56 (234) 94 (393) 85 (356) CH;CH,CH,- 101 (423) 110 (460) 85 (356) 70 (293) 56 (234) 92 (385) 84 (352) (CH;),CH- 98.5 (412) 111 (464) 84 (352) 71 (297) 56 (234) 96 (402) 86 (360) (CH3);C- 96.5 (404) 110 (460) 85 (356) 71 (297) 55 (230) 96 (402) 85 (356) Note: (a) DH° = AH° for the process A-B → A + ·B. (b) These numbers are being revised continually because of improved methods for their measurement. (c) The trends observed for A-H bonds are significantly altered for polar A-B bonds because of dipolar contributions to DH°.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
The H values for the addition of each of the molecules to ethene can be calculated by taking into account the bond energies of the reactants and produ...View the full answer

Answered By

DHRUV RAI
As a tutor, I have a strong hands-on experience in providing individualized instruction and support to students of all ages and ability levels. I have worked with students in both one-on-one and group settings, and I am skilled in creating engaging and effective lesson plans that meet the unique needs of each student.
I am proficient in using a variety of teaching techniques and approaches, including problem-based learning, inquiry-based learning, and project-based learning. I also have experience in using technology, such as online learning platforms and educational software, to enhance the learning experience for my students.
In addition to my teaching experience, I have also completed advanced coursework in the subjects that I tutor, including mathematics, science, and language arts. This has allowed me to stay up-to-date on the latest educational trends and best practices, and to provide my students with the most current and effective teaching methods.
Overall, my hands-on experience and proficiency as a tutor have equipped me with the knowledge, skills, and expertise to help students achieve their academic goals and succeed in their studies.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Calculate Ho values for the following reactions. (a) H2 + F2 2 HF; (b) H2 + Cl2 2 HC1; (c) H2 + Br2 2 HBr; (d) H2 + I2 2 HI; (e) (CH3)3CH + F2 (CH3)3CF + HF; (f) (CH3)3CH + Cl2 (CH3)3CCl + HC1;...
-
For this assignment you just have to report the values of Timer 0 registers TCNTO, TCCROA & B for the two simple questions. Q1. To create a 20us square wave with the help of 10 MHz clock, what will...
-
The Fitness Studio, Inc., with the help of its investment bank, recently issued $43.125 million of new debt. The offer price (and face value) on the debt was $1,000 per bond and the underwriters...
-
Q3: Prove: For any sets A and B, Ax B = B A ?
-
Explain what is meant by crisis management and why many organizations continue to exist in this type of environment.
-
During embryonic development, what features do we share with tunicates or lancelets?
-
I:5-67 Short Tax Form. Given the following information for Jane Cole, complete Schedule D of Form 1040 through Part III. Stock options, which she purchases on February 14 of the current year for...
-
Which of the four duties that Blatt owed Scott in their agency relationship has probably been breached? James Blatt hired Marilyn Scott to sell insurance for the Massachusetts Mutual Life Insurance...
-
On September 1, 2019, Donna Equipment signed a one-year, 8% interest bearing note payable for $100,000. Assuming Donna maintains its books on a calendar year basis, the amount of interest expense...
-
Meredith Merriweather, CPA is the CFO of Trego Bikes and Trikes (TBT), a manufacturer of Bicycles ranging from tricycles to high-end racing bikes. The company has good market penetration and has seen...
-
What is the IUPAC name for compound B (see margin)? (a) (E)-2-Methyl-3-pentene; (b) (E)-3-methyl-2-pentene; (c) (Z)-2-methyl-3-pentene; (d) (Z)-3-methyl-2-pentene
-
The bicyclic alkene car-3-ene, a constituent of turpentine, undergoes catalytic hydrogenation to give only one of the two possible stereoisomeric products. The product has the common name cis-carane,...
-
Show in a drawing similar to Figure 18.18 how two different programs with the same logical address space can be transformed by virtual storage partially into the same part of physical memory and...
-
Discuss charitable purpose trusts under Section 3(1), Charities Act 2011.
-
Amadeus Corporation is considering the issue of a new product to be added to its product mix. They hired you, a recent business graduate from MacEwan, for conducting the analysis. The production line...
-
I have attached a case study, primarily based on your textbook chapter reading assignments. The background material for the case also references chapters 3 and 15, not assigned for this course....
-
On December 1 , 2 0 2 5 , Sandhill Distributing Company had the following account balances.DebitCash$ 7 , 1 0 0 Accounts Receivable 4 , 5 0 0 Inventory 1 1 , 9 0 0 Supplies 1 , 2 0 0 Equipment 2 2 ,...
-
Cindy Greene works at Georgia Mountain Hospital. The hospital experiences a lot of business closer to summer when the temperature is warmer. Cindy is meeting with her supervisor to go over the budget...
-
Write a program that produces a Caesar cipher of a given message string. A Caesar cipher, or rotation cipher, is formed by rotating each letter of a message by a given amount. For example, if you...
-
1. Advertising for eyeglasses _________ (increases/decreases) the price of eyeglasses because advertising promotes _________. 2. An advertisement that succeeds in getting consumers to try the product...
-
Find an expression for the fugacity coefficient of a gas that obeys the equation of state pVm = RT(1 +B/Vm + C/V-1). Use the resulting expression to estimate the fugacity of argon at 1.00 am3 and 100...
-
At 298 K the standard enthalpy of combustion of sucrose is -5797 k] mol-I and the standard Gibbs energy of the reaction is -6333 k] mol ". Estimate the additional non-expansion work that may be...
-
In 1995, the Intergovernmental Panel on Climate Change (IPCC) considered a global average temperature rise of 1.0-3SC likely by the year 2100, with 2.0C its best estimate. Because water vapour is...
-
1-5 Apps M Gmail o YouTube Maps OL ch6-3 Saved Menlo Company distributes a single product. The company's sales and expenses for last month follow Per Unit $2e points Sales Variable expenses...
-
The following information relates to an equipment lease with an inception date of January 1: Fair value of equipment at lease inception, $56,000 Lease term, 5 years Economic life of property, 6 years...
-
Add since or for question number first he has been selling cars _ ten year

Study smarter with the SolutionInn App


