The commercial synthesis of a useful heterocyclic derivative requires treatment of a mixture of aldopentoses (derived from
Question:
The commercial synthesis of a useful heterocyclic derivative requires treatment of a mixture of aldopentoses (derived from corncobs, straw, etc.) with hot acid under dehydrating conditions. The product, E, has 1H NMR spectrum E, shows a strong IR band at 1670 cm-1, and is formed in nearly quantitative yield. Identify compound E and formulate a mechanism for its formation.
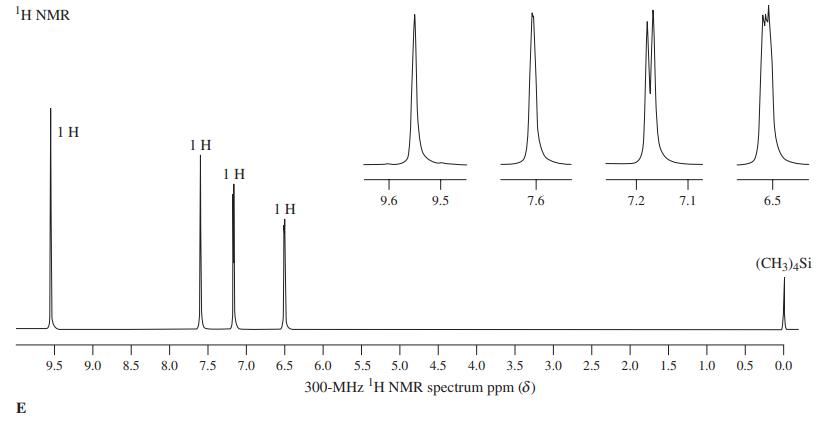
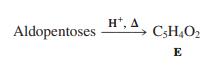
Compound E is a valuable synthetic starting material. The following sequence converts it into furethonium, which is useful in the treatment of glaucoma. What is the structure of furethonium?
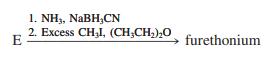
Treatment of a 3-acylindole with LiAlH4 in (CH3CH2)2O reduces the carbonyl all the way to a CH2 group. Explain by a plausible mechanism.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





