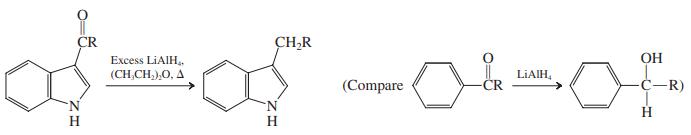
Treatment of a 3-acylindole with LiAlH 4 in (CH3CH2)2O reduces the carbonyl all the way to a
Question:
Treatment of a 3-acylindole with LiAlH4 in (CH3CH2)2O reduces the carbonyl all the way to a CH2 group. Explain by a plausible mechanism.
Transcribed Image Text:
CR Excess LIAIH, (CH,CH,),0, A CH,R OH LIAIH, (Compare CR -C-R) H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
O 11 CR OLI ...View the full answer

Answered By

Vidyasagar KUSHWAHA
Presentl i have been cleared CSIR NET/JRF examination which is cetral level exam .I have also written IIT GATE examination whose result in waiting till now.
I have been granted CSIR JRF and now i am preparing for interview for Ph.D. admission
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
The commercial synthesis of a useful heterocyclic derivative requires treatment of a mixture of aldopentoses (derived from corncobs, straw, etc.) with hot acid under dehydrating conditions. The...
-
Carboxylic acids having a second carbonyl group two atoms away lose CO2 (decarboxylate.) through an intermediate enolate ion when treated with base. Write the mechanism of this decarboxylation...
-
Proteins can be cleaved specifically at the amide bond on the carboxyl side of methionine residues by reaction with cyanogen bromide, BrC = N. The reaction occurs in several steps: (a) The first step...
-
M/s Active Builders Ltd. invested in the shares of another company (with an intention to hold the shares for short term period )on 31st October, 2016 at a cost of Rs.4,50,000. It also earlier...
-
(i) In the model with one endogenous explanatory variable, one exogenous explanatory variable, and one extra exogenous variable, take the reduced form for y2, (15.26), and plug it into the structural...
-
Identify and discuss the ways discharge may be brought about by operation of law.
-
Describe how the SDLC pertains to the development of this application. Describe the tasks that need to be accomplished in each phase. Indicate who should perform the tasks: MRV, an outsource...
-
The bank portion of the bank reconciliation for Katsaris Company at August 31, 2014, was as follows: The adjusted cash balance per bank agreed with the cash balance per books at August 31. The...
-
Express the confidence interval 29.7%3.1% in the form of a trilinear inequality. %
-
Taussig Technologies Corporation (TTC) has been growing at a rate of 20% per year in recent years. This same supernormal growth rate is expected to last for another 2 years (g1 = g2 = 20%). a. If D0...
-
Heterocycle C, C 5 H 6 O, exhibits 1 H NMR spectrum C and is converted by H 2 and Raney nickel into compound D, C 5 H 10 O, with spectrum D. Identify compounds C and D. (Note: The coupling constants...
-
The sequence in the margin is a rapid synthesis of one of the heterocycles in this chapter. Draw the structure of the product, which has 1 H NMR spectrum F. 'H NMR 1 H 1 H 3 H 1H. 9.2 8.5 8.4 7.9 7.8...
-
If the coefficient of static friction at A is ? s = 0.4 and the collar at B is smooth so it only exerts a horizontal force on the pipe, determine the minimum distance x so that the bracket can...
-
Check that [edgm \(][\) cdgm \(]\) is a graphical model. Education Gender MS Dep CIRS
-
Use the information provided in P3-9B. Required a. Prepare closing entries at December 31 in general journal form using the Income Summary account. b. After the closing entries are posted, calculate...
-
If the light bulb in Figure \(33.8 a\) is \(1.0 \mathrm{~m}\) in front of the mirror, how far behind the mirror is the image? Data from Figure 33.8a (a) Rays shows path of light that travels from...
-
\(300.0 \mathrm{kmol} / \mathrm{h}\) of a saturated liquid feed that is \(40.0 \mathrm{~mol} \% \mathrm{n}\)-nonane (C9) and \(60.0 \mathrm{~mol} \% \mathrm{n}\)-decane (C10) at \(11.0 \mathrm{kPa}\)...
-
Show that for a Hamiltonian of the form \[H=-G \sum_{m, m^{\prime}>0} a_{m^{\prime}}^{\dagger} a_{-m^{\prime}}^{\dagger} a_{-m} a_{m}\] the energy eigenvalues for the quasispin model of Problem 31.1...
-
Add a method bubbleSort to the class ArraySorter, as given in Listing 7.10, that performs a bubble sort of an array. The bubble sort algorithm examines all adjacent pairs of elements in the array...
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
The following 1H NMR spectrum is that of an alcohol, C8H10O. Propose a structure. TMS 6. Chemical shift (8) 3 O ppm 10 8. Intensity
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C5H12O (b) C8H10O Part (a) TMS O ppm 10 3 2 Chemical shift (8) Part (b) TMS O ppm 10 8. 6. 4 3 2 Chemical shift (8) Inten
-
Propose structures for alcohols that have the following 1H NMR spectra: (a) C9H12O (b)C8H10O2 Part (a) TMS 10 O ppm Chemical shift (8) Part (b) TMS O ppm 10 8. Chemical shift (8) Intensity Intensity...
-
Content Area The following accounts appear in the ledger of Monroe Entertainment Co. All accounts have normal balances. Accounts Payable $471 Fees Earned $2,347 Accounts Receivable 741 Insurance...
-
Concord Importers provides the following pension plan information. Fair value of pension plan assets, January 1, 2020 $2,192,000 Fair value of pension plan assets, December 31, 2020 2,549,000...
-
CELL PHONE USAGE NOT ALLOHC Company's accounts receivable for 2024: 7. The following information relates to Acme Compa

Study smarter with the SolutionInn App


