The Hell-Volhard-Zelinsky reaction produces only bromocarboxylic acids. However, modifi ed procedures have been developed to convert acyl
Question:
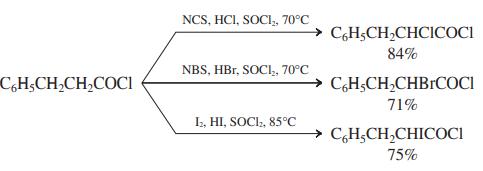
The Hell-Volhard-Zelinsky reaction produces only bromocarboxylic acids. However, modifi ed procedures have been developed to convert acyl chlorides into a-chloro- and a-bromoacyl chlorides by reaction with N-chloro- and N-bromobutanimide (N-chloro- and N-bromosuccinimide, NCS and NBS), respectively. Reaction with I2 gives α-iodoacyl chlorides. (SOCl2 is used as the solvent to maintain the acyl chloride functional group.) Suggest a mechanism for any one of these processes.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





