The removal of the C17 side chain of certain steroids is a critical element in the synthesis
Question:
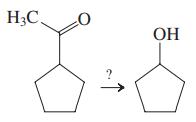
The removal of the C17 side chain of certain steroids is a critical element in the synthesis of a number of hormones, such as testosterone, from steroids in the relatively readily available pregnane family.
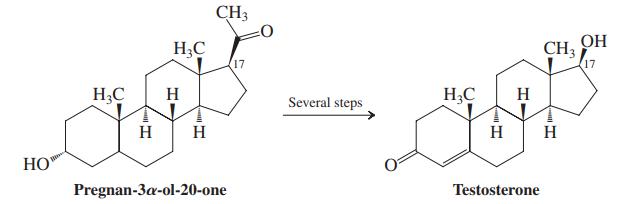
How would you carry out the comparable transformation, shown in the margin, of acetylcyclopentane into cyclopentanol?
Transcribed Image Text:
CH3 H;C (17 OH CH3 17 H3C H Several steps H;C H H H H H HO Pregnan-3a-ol-20-one Testosterone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
The removal of the acet yl ...View the full answer

Answered By

Gilbert Chesire
I am a diligent writer who understands the writing conventions used in the industry and with the expertise to produce high quality papers at all times. I love to write plagiarism free work with which the grammar flows perfectly. I write both academics and articles with a lot of enthusiasm. I am always determined to put the interests of my customers before mine so as to build a cohesive environment where we can benefit from each other. I value all my clients and I pay them back by delivering the quality of work they yearn to get.
4.80+
14+ Reviews
49+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
How would you carry out the followingreactions? (a) CCH-CCH3 CH3CH2C=CH () H2C%3CH CH3CH2CH2CHO .3- (c) 3C (d) CH . CH 7, 22H (e) CH3CH2C=CH (f) CH3CH2CH2CH2CH=CH2 CHCH2CH2CH2C%CH (2 steps)
-
How would you carry out the followingsyntheses? Cyclohexene Cyclohexanol Cyclohexane ~/~/al
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
Ten thousand dollars is deposited in a savings account at 4.6% interest compounded continuously. When will the balance triple? O within a year of investment. O In 10 years approximately. none of the...
-
How does your answer in Problem 7 change if Finland has 3 million workers instead of 1.5 million? Answer verbally; no computation is needed. Consider the following model of trade between Iceland and...
-
The database Dish.xlsx contains a transaction history describing more than 4,000 purchases of detergent at a number of stores in a grocery chain over a period of several weeks. a. Compile a pivot...
-
Instructions Obtain the most recent edition of Accounting Trends and Techniques. Examine the disclosures included under the section regarding gain contingencies, and answer the following questions....
-
California Surf Clothing Company issues 1,000 shares of $1 par value common stock at $35 per share. Later in the year, the company decides to repurchase 100 shares at a cost of $38 per share. Record...
-
Silven Industries, which manufactures and sells a highly successful line of summer lotions and insect repellents, has decided to diversify in order to stabilize sales throughout the year. A natural...
-
To determine the prevalence of human growth hormone (HGH) use among high school varsity baseball players, the State Athletic Commission randomly selects 50 high schools. All members of the selected...
-
Show how you would carry out the following transformation in which the ester function at the lower left of the molecule is converted into a hydroxy group but that at the upper right is preserved. (Do...
-
Propose a synthetic sequence to convert carboxylic acid A into the naturally occurring sesquiterpene -curcumene. CH3 CH3 CO,H CH3 CH3 H3C CH3 A a-Curcumene
-
The front of a jet engine acts as a diffuser receiving air at 900 km/h, -5C, 50 kPa, bringing it to 80 m/s relative to the engine before entering the compressor. If the flow area is reduced to 80% of...
-
The revenue recognition principle and the expense recognition principle require that the company recognize related revenue and expense transactions in the same accounting period. Discuss why this...
-
Revisits scope, time, and cost baselines in the context of agile methodologies. Because agile includes several methodologies (like Scrum, Kanban, Extreme Programming, Feature-Driven Development) we...
-
Background information and task: Fed officials divided in July over need for more rate hikes, minutes show WASHINGTON, Aug 16 (Reuters) - Federal Reserve officials were divided over the need for more...
-
Questions: 1. What are the long-term prospects for the Chinese market? 2. Does it make sense for GM to produce automobiles for the Chinese market in China? Why? 3. What do you think would happen if...
-
The purpose of this assignment is to apply your knowledge of conflict management to a real-world situation so that you can enhance your skills in handling conflicts. It is crucial to carefully read...
-
Outline mechanisms that might be used by a binary rewriter, without access to source code, to catch uses of uninitialized variables, double deletes, and uses of deallocated memory (e.g., dangling...
-
Give the structural formulas of the alkenes that, on ozonolysis, give: a. (CH3)2C=O and CH2=O b. Only (CH3CH2)2C=O c. CH3CH=O and CH3CH2CH=O d. O=CHCH2CH2CH2CH=O
-
The reaction of an alkyl chloride (or bromide) with sodium iodide in acetone proceeds according to the following equation: Sodium iodide is soluble in acetone, whereas both sodium chloride and sodium...
-
The Lucas test is used to check for the presence of an alcohol functional group in an unknown compound. The test reaction is shown in the following equation: Smaller alcohols are soluble in the...
-
Explain why this secondary alcohol reacts with HCl and ZnCl2 in H2O at about the same rate as a primary alcohol (see Problem 8.38) OH Cl-CH,CHCH3
-
Joseph Industries manufactures wooden backyard playground equipment. Joseph estimated $1,815,000 of manufacturing overhead and $2,160,000 of direct labor cost for the year. After the year was over,...
-
.Passive loss rules do NOT apply to a limited partner who meets which of the following material participation tests? Participation for more than 500 hours in the activity during the year. Material...
-
Iwrite a short paragraph explaining your Royal bank checking account describe the prospect

Study smarter with the SolutionInn App


