Using methods reverse polarization, propose a simple synthesis of each of the following molecules. (a) (b) (c)
Question:
Using methods reverse polarization, propose a simple synthesis of each of the following molecules.
(a)
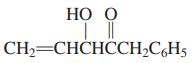
(b)
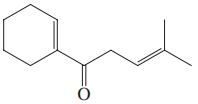
(c)
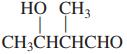
Transcribed Image Text:
НО О CH2=CHCHCCH2C,H5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
The given conversions are car...View the full answer

Answered By

JAIPRATAP SINGH SONU
My expertise is chemistry, mostly organic chemistry. This involves synthesizing, characterizing, and identifying organic molecules. I'll guide you towards best resources for learning key concepts that'll make you self-sufficient. You can expect the following : 1) Use of standard text books. 2) Use of peer- Reviewed Journals. 3) Use of reputed institute's resources. 4) Timely help for assignments and projects. 5) Help in learning key concepts. The solutions would be provided in pdf or word format within promised time, without any delay. There is a difference between solutions provided for Home-Work problems and solutions for extra credit assignments. Higher quality solutions with references would cost more but their sterling quality would be guaranteed, on the face of it. You would be able to trace back, the resources used for solutions provided to you.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
For each of the following molecules, propose two methods of synthesis from the different precursor molecules indicated. (a) CH 3 CH=CHCH 2 CH(CH 3 ) 2 from (1) an aldehyde and (2) a different...
-
Propose a synthesis for each of the following compounds, using a Robinson annulations: a. b. c. d. CH CH3 CH3 CHs H-C
-
Propose an efficient synthesis for each of the following compounds using the acetoacetic ester synthesis. (a) (b) (c) (d)
-
41.(6 pointa) In the following balanced reaction, magnesium metal (Mg) reacts with carbon dioxide gas (CO) o form solid magnesium exide (MgO) and solid carbon (C). How many grams of carbon dioxide...
-
Explain how a supply curve can be obtained or derived from an increasing cost production possibility curve. Use Figure 4.3 to derive the supply curve for cloth. For a bit more challenge, use Figure...
-
Why is multiplication of matrices restricted by conditions on the factors?
-
7. (a) or (b) If x = limn~oo Xn exists, what is limn~oo X n+l?
-
Target costs, effect of product-design changes on product costs. Medical Instruments uses a manufacturing costing system with one direct-cost category (direct materials) and three indirect-cost...
-
Dove Corporation began its operations on September 1 of the current year. Budgeted sales for the first three months of business are $246,000, $307,000, and $413,000, respectively, for September,...
-
Preble Company manufactures one product. Its variable manufacturing overhead is applied to production based on direct labor-hours and its standard cost card per unit is as follows: Direct material: 4...
-
A short construction of the steroid skeleton (part of a total synthesis of the hormone estrone) is shown here. Formulate mechanisms for each of the steps. 6. CH, O: KOH, CH,OH, A CH;0 CH, CH3 HC...
-
Propose a synthesis of ketone C, which was central in attempts to synthesize several antitumor agents. Start with aldehyde A, lactone B, and anything else you need. H,C H2C A B C
-
During 2016, Northwest Satellite Systems, Inc., purchased two other companies for $13 million in cash. Also during 2016, Northwest made capital expenditures of $11.3 million in cash to expand its...
-
1. Using the net present value? method, calculate the comparative cost of each of the three payment plans being considered by New Med 2. Which payment plan should New Med choose? Explain. 3. Discuss...
-
Swenson Company produced 300 units in year one and sold 260 units in that year. In year two, it produced 260 units and sold 300 units. Total fixed overhead was the same in years one and two. Under...
-
c) Determine the maximum rotational speed such that the fluid will not spill over the container. (and: = 2gh/R) [2 marks] d) The container in Figure 4 now contains coffee (p~1000) which is 7cm deep...
-
FICO credit scores: x = 564,= 743,= 72 (Round your answer to 3 decimal places.) what does z equal
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28 W/m. 'C. The thermal conductivity of the solid material is...
-
Suppose that you are trying to write a program that produces the following output: 1 3 5 7 9 11 13 15 17 19 21 1 3 5 7 9 11 The following program is an attempt at a solution, but it contains four...
-
Gordon and Lisa estimate that they will need $1,875,000 in 40 years for their retirement years. If they can earn 8 percent annually on their funds, how much do they need to save annually?
-
Tell the number of hydrogens bonded to each carbon atom in the following substances and give the molecular formula ofeach: OH H (a) (b) CO2CH3 Ephedrine Cocaine
-
Identify the most electronegative element in each of the following molecules: (a) CH2FC1 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
More info
-
Andretti Company has a single product called a Dak. The company normally produces and sells 8 0 , 0 0 0 Daks each year at a selling price of $ 5 6 per unit. The company s unit costs at this level of...
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...

Study smarter with the SolutionInn App


