What product(s) would form if each carbonyl compound in Problem 32 were treated with (a) alkaline D
Question:
What product(s) would form if each carbonyl compound in Problem 32 were treated with (a) alkaline D2O; (b) 1 equivalent of Br2 in acetic acid; (c) excess Cl2 in aqueous base?
Data From Problem 32
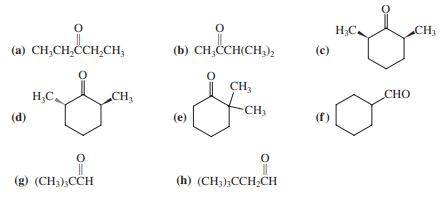
Transcribed Image Text:
H;C. CH3 (a) CH;CH,ČCH,CH, (b) CH,CCH(CH,h (c) CH, H,C. CH3 CHO CH, (d) (e) (g) (CH3);CCH (h) (CH3),CCHCH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
What product(s) would you expect from the methanolysis of the iodocyclohexane derivative given as the reactant in Practice Problem 6.8? In Problem 6.8 CH3 (CH3)3 I H20 SN1
-
What product(s) would you expect from reaction of (S)-3-chloro-3-methyloctane with acetic acid? Show the stereo chemistry of both reactant and product.
-
(a) Give the structure of cocaine (Fig. 23.4) as it would exist in 1 M aqueous HCI solution. (b) What products would form if cocaine were treated with an excess of aqueous NaOH and heat? (c) What...
-
Now apply one of those decision making models (Philosophical) to this scenario: Scenario: You are a CPA in the accounting department at a HealthCare System with three other accountants. You are good...
-
How is the right of publicity different from the right of privacy? See the Elvis Presley case in this chapter.
-
Modify the CreditCard class from Code Fragment 1.2 to charge a late fee for any payment that is past its due date. Data from in Fragment 1.2 A typical C++ program includes many different header...
-
1 2 Give examples showing how the context, including the psychological contract, affects motivation
-
An inspector samples cans from a truckload of canned creamed corn to estimate the total number of worm fragments in the truckload. The truck has 580 cases; each case contains 24 cans. The inspector...
-
Harry vendi una propiedad de uso personal por $3000 que haba comprado por $4000 y que haba tenido durante varios aos. Qu tratamiento fiscal recibe con respecto a su prdida
-
Which 3 statements are correct regarding reconciling a bank account in QuickBooks Online? To begin the reconcile process, you need to enter the statement ending date and ending balance from the...
-
Write the structures of every enol and enolate ion that can arise from each of the carbonyl compounds illustrated in Problem 32. Data From Problem 32 H;C. CH3 (a) CH;CH,CH,CH3 (b) CH;CCH(CH3)2 () CH3...
-
Describe the experimental conditions that would be best suited for the efficient synthesis of each of the following compounds from the corresponding nonhalogenated ketone. (a) (b) (c) Br O CH;CHCCH3
-
A spring whose equilibrium length is 15 cm exerts a force of 50 N when it is stretched to 20 cm. Find the work required to stretch the spring from 22 to 24 cm.
-
Discuss how technology and human resources are needed to operate this facility in this behind the scenes look at this retailing giant. Support your opinion with research and/or key concepts covered...
-
4.2 At a given instant, a spacecraft is 500 km above the earth, with a right ascension of 300 and a declination of -20 relative to the geocentric equatorial frame. Its velocity is 10 km/s directly...
-
A 447 gram cart (mA) slides along a very smooth track and collides with a stationary 475 gram cart (mB). A motion detector records the velocity of cart A, as shown in Figures 1 and 2. A force probe...
-
M8 Homework i Saved 1 Mayfair Company completed the following transactions and uses a perpetual inventory system. Help Save & Exit Submit Check my work 10 points eBook Print References June 4 Sold...
-
Free Response Table Problem x -6 -80 -4 -3 f(x) 1.948 1 0 -2 -2.005 -798 undefined -2 -1.995 0 1 1.995 2 2.005 6 80 802 4 3.333 3.001 undefined 2.998 2.5 2.048 23. The table above represents values...
-
Listed here are the stockholders equity sections of three public companies for fiscal years ending in 2017 and 2016. (Note that for General Mills these data are for the fiscal years ended on May 27,...
-
You purchase a bond with a coupon rate of 6.7 percent, a par value $1,000, and a clean price of $905. Assume a par value of $1,000. If the next semiannual coupon payment is due in two months, what is...
-
The enzyme-catalyzed conversion of a substrate at 25C has a Michaelis constant of 0.042 mol dm-3. The rate of the reaction is 2.45 x 10-4 mol dm-3 s-1 when the substrate concentration is 0.890 mol...
-
In a photochemical reaction A -7 B + C, the quantum efficiency with 500 nm light is 1.2 x 102 mol einstein-1, After exposure of 200 mmol A to the light, 1.77 mmol B is formed. How many photons were...
-
In an experiment to measure the quantum efficiency of a photochemical reaction, the absorbing substance was exposed to 320 nm radiation from an 87.5 W source for 28.0 min. The intensity of the...
-
Report and evaluate the cash position of Microsoft for the past 2 years based on your understanding of the report. Provide a detailed rationale on whether you would lend money to this company if you...
-
Calculate the average of all the elements of column with name List such that the number of Baths is less than 4. Place the answer in cell L6. Download file StudentQ5.xlsx from below: - StudentQ5.xlsx...
-
Confused as to what would go in the General Journal if there is only 4 slots Marydale Products permits its customers to defer payment by giving personal notes instead of cash. All the notes bear...

Study smarter with the SolutionInn App


