Write the most reasonable structure of the product of each of the following reaction sequences. (a) (b)
Question:
Write the most reasonable structure of the product of each of the following reaction sequences.
(a)
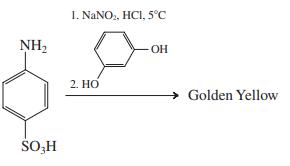
(b)
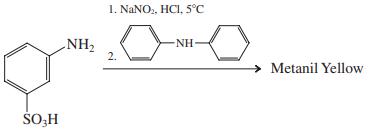
For the following reaction, assume that electrophilic substitution occurs preferentially on the most activated ring.
(c)
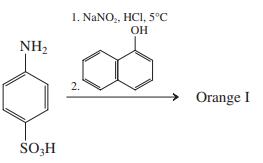
Transcribed Image Text:
I. NANO, HCI, 5°C NH2 OH 2. HO Golden Yellow SO,H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
a b c How electrophilic is produced ...View the full answer

Answered By

Appala naidu karri
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as neede
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the expected product of each of the following reaction sequences. (a) (b) (c) H 1. LIAIH,, (CH,CH,),O 2. H., . H
-
Give the product(s) of the following reaction sequences. 1. , * 2. 1. , * 2. (CH),CH.CI 3. ', . CH,Br 3. H., . () CH,CO (b) CH2CHO
-
Give the expected product(s) of each of the following reaction sequences. (a) (b) (c) (d) (e) (e) 1.2 CH, Br, NaOH 2.
-
Four of Wands, LLC generated $255,000 in sales during January 2022. Of this amount, 25% was for cash. The remaining 75% of sales were made on account. The February 2022 sales on account were...
-
The minister for labor of the small nation of Pembangunan is eager to encourage domestic production of digital clocks. A small clock industry exists, but only a few producers can survive foreign...
-
Dont confuse Cauchys integral theorem (also known as CauchyGoursat theorem) and Cauchys integral formula. State both. How are they related?
-
6.4 Alternating Series 1. (d) Use Example 6.34. 2. (a) [-1,1). (b) (-0,0). (c) (-1,1]. (d) [-3, -1]. 3. (a) Absolutely convergent. (b) Absolutely convergent. (c) Absolutely convergent. (d)...
-
1. How can the management specifically improve the stewarding process at The Phoenician? Using the information provided, create a flowchart illustrating the new process. 2. What are the benefits that...
-
Mary Janes cumulative year-to-date earnings are $7,200. On June 30th, Mary Jane earned $600. Presuming the FUTA tax rate is 0.6% and a ceiling of $7,000, what is the FUTA tax amount that Mary Janes...
-
Although organizations in the manufacturing sector usually carry more inventory than service firms, effective inventory management is nonetheless important to both manufacturers and service...
-
Devise a synthesis of each of the following substituted benzene derivatives, starting from benzene. (a) (b) (c) (d) (e) (f) (g) Br
-
Show the reagents that would be necessary for the synthesis by diazo coupling of each of the following three compounds. (a) Methyl Orange (b) Congo Red (c) -SO,NH2 Prontosil, H2N- which is NH2...
-
Find a summary of the Daubert v. Merrell Dow Pharmaceuticals court case. Identify five criteria a trial judge should consider when evaluating ex-pert testimony. Prepare a memo to your instructor...
-
1. Implement the function of a XNOR gate by a 2 to 4 decoder. Use logic gates if needed at the output. 2. The following question is to design an octal to binary encoder. a) Write down the truth table...
-
# III: Worksheet 3 1. A 20 kg mass is allowed to accelerate down a frictionless 15 ramp. 20 kg 15 a. Draw a force diagram for the block. b. Determine the value of the x-component of the force of...
-
3.Baker Corporation has provided the following production and average cost data for two levels of monthly production volume. The company produces a single product Production Volume: 1,000 units:...
-
Suppose that you own the only company in the market to produce a certain product, and therefore you can determine the market price P dollars for each unit. Due to government regulations, the price of...
-
describes how the blast pressure front can bounce off solid, immovable obstacles and be redirected in another direction in a linear angle to the angle of the obstacle hat was struck
-
Write nested for loops to produce the following output: * ** *** **** *****
-
When an electric field is applied to a shallow bath of vegetable oil, why do tiny bits of thread floating in the oil align with the field like compasses in a magnetic field?
-
Predict the relative intensities of the M +, M + 2, and M + 4 peaks for these compounds. Assume that 79Br/81Br = 1/1 and 35Cl/37Cl=3/1. (a) CH2Cl2 (b) CH2BrCl
-
What conclusions can be drawn about these compounds from their massspectra? 100 43 55 135 85 69 107 164 trpt 40 50 60 70 80 L10 120 130 90 100 140 150 160 170 m/z b) 100 73 50 20 30 40 50 60 70 80 90...
-
Show the molecular ions formed from these compounds: b) a) CH,NHCH,
-
According to Barth, Caprio, and Levine, regulators ought to think of ways of helping financial markets, particularly bank debt and equity holders, to monitor banks. 1) True 2) False VE
-
Suppose you see that a stock has a very high Price-to-earnings (P/E) ratio. Does it imply that this stock is overvalued? Why or why not? Explain
-
Statements on Auditing Standards (SAS) provide more detailed guidance of: a. PCAOB assertions b. statutory law c. GAAS d. GAAP

Study smarter with the SolutionInn App


